Seed Oils, Early-Onset Cancer & Human Metabolic Variability
Early-onset breast and colon cancer are on the rise. Are seed oils one of the modern environmental factors contributing to this?
Not medical advice.
This content draws from two M&M podcasts that focus on 2025 studies linking ω-6 polyunsaturated fatty acids (PUFAs) to cancer. The episodes and studies discussed are listed below.
Colon cancer:
Podcast: Dietary Fats & Seed Oils in Inflammation, Colon Cancer & Chronic Disease | Tim Yeatman & Ganesh Halade
Study: Integration of lipidomics with targeted, single cell, and spatial transcriptomics defines an unresolved pro-inflammatory state in colon cancer
Breast cancer:
Podcast: Linoleic Acid, Seed Oils, mTOR & Breast Cancer | Nikos Koundouros & John Blenis
Study: Direct sensing of dietary ω-6 linoleic acid through FABP5-mTORC1 signaling

One of the more alarming trends we see today is that certain cancers are becoming more common, with some rising fastest among younger people.
Why would this be?
The traditional and still dominant view of oncogenesis (how cancer starts) is that cancer is primarily caused by the accumulation of mutations in the nuclear genome. Over time, exposure to carcinogens results in DNA mutations in key genes (oncogenes or tumor suppressor genes), which are often important for cell cycle regulation. When they mutate, the process of cell division breaks free of its normal constraints, resulting in cancer.
An alternative view promoted by cancer biologist Dr. Thomas Seyfried is gaining traction: cancer is fundamentally a metabolic disease, more than it is a genetic one. In other words, cells tend to become cancerous due to metabolic dysregulation rooted in mitochondrial function, rather than from accumulation of mutations in the nuclear genome.
To learn more about cancer metabolism and its roots in mitochondrial biology, listen to my conversation with Dr. Seyfried. No matter what particular view you take of oncogenesis, there is no doubt that the nutrients we consume influence whether cancer arises and how it progresses.
This post will highlight new evidence that points to omega-6 polyunsaturated fatty acids (ω-6 PUFAs) as a driver of two cancers that are on the rise in younger people: colon and triple-negative breast cancer. What’s even more concerning is that the molecular details for how this seems to be happening suggests that ω-6 PUFAs will almost be certainly linked to other forms as cancer as well.
ω-6 PUFAs are the major component of seed oils. Unless otherwise noted, you can take “seed oils” to be synonymous with “ω-6 PUFAs” here. Seed oils are just one prominent component of the modern food environment, which is just one aspect of the overall environment we’re embedded in.
To be clear: there are certainly other environmental factors contributing to modern trends in cancer specifically and chronic disease generally. These range from overconsumption of other nutrients (e.g. fructose) to synthetic pesticides, endocrine disruptors, artificial light, and more.
Learn more about environmental factors that drive cancer and metabolic dysfunction:
Podcast | Cancer Metabolism: Sugar, Fructose, Lipids & Fasting | Gary Patti
Article, Podcast | Glyphosate, Choline, Brain Inflammation & Neurodegeneration
Podcast | Regenerative Energy & the Light Inside You | Jack Kruse
Below, we will review new research on the link between ω-6 PUFAs (linoleic acid in particular) and two forms of cancer. After that, I will discuss important aspects of human metabolic variability that affect people’s “tolerance” to ω-6 PUFAs. This variability is rarely (if ever) accounted for in human clinical trials, obscuring our ability to recognize the negative health consequences of ω-6 PUFAs and confusing people who take the clinical literature at face value without properly considering shortcomings in study design and data analysis.
I recommend looking at this content as needed if you lack a solid foundation in PUFA biology, fat metabolism, and historical trends in dietary fat intake:
Content that will help you better understand this article.
Article | Eating Fat Like Never Before
Article | PUFAs & The Palisades: Lipid Peroxidation, Oxidative Stress & Cell Death
Podcast | Seed Oils, Omega-6 PUFAs, Inflammation, Obesity, Diabetes, Chronic Disease & Metabolic Dysfunction | Chris Knobbe
Podcast | Omega-6-9 Fats, Seed Oils, Metabolic Health & Chronic Inflammatory Disease | Artemis Simopoulos
Colon Cancer: PUFAs & Gut Inflammation
Here is a study highlighting that colon cancer is on the rise in much of the world, especially among younger people:
Early-onset colorectal cancer incidence rates are rising in 27 of 50 countries and territories examined, with the rise either exclusive to early-onset disease or faster than the increase in older adults in 20 of the 27 countries.
And here’s what the data look like for a selection of countries, including the US, comparing younger (25-49) to older people (50-74) from 1975 to the 2010s.
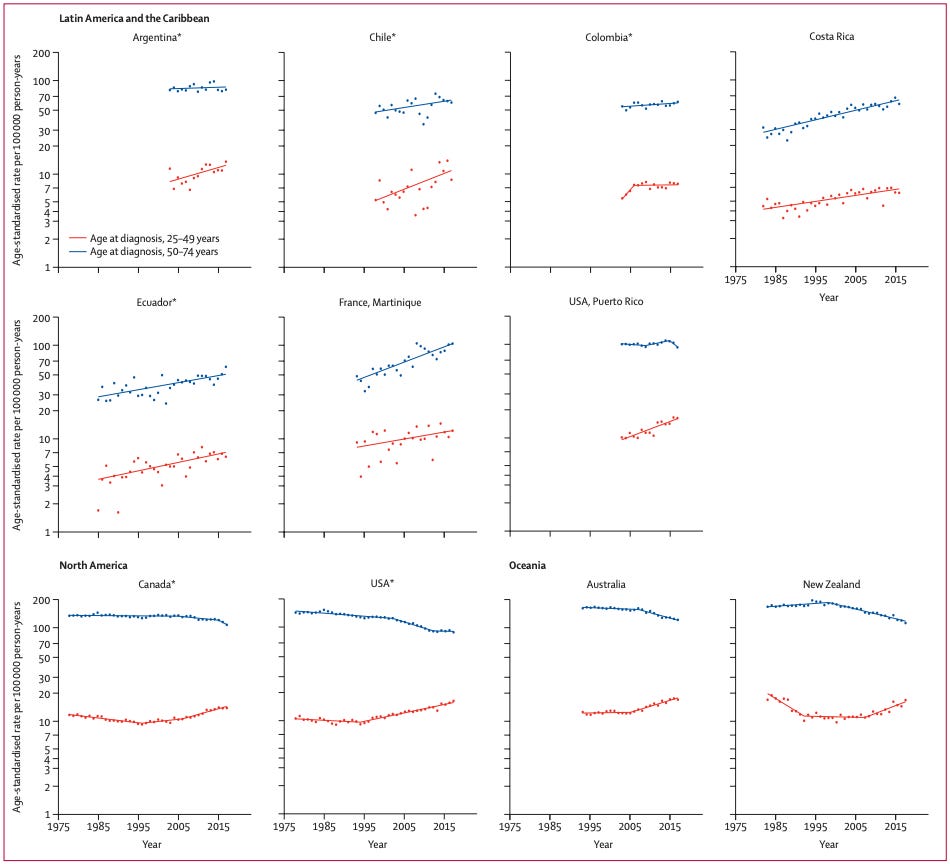
In many countries, colon cancer is on the rise among the younger cohort. For the older cohort, the pattern varies by country. Notice that in places like the US, colon cancer rates have been increasing for the younger cohort since the mid-1990s, despite a clear drop in rates for the older cohort happening at the same time.
Why would this be the pattern? Cancer is clearly associated with age—the longer you’re alive, the more time there is for your cells to become dysregulated, no matter the cause. Cancer is more common overall later in life, so why would colon cancer be on the rise in younger people while simultaneously falling in older people?
In M&M 200, I talked with two scientists who ran a lipidomics study linking an inflammatory tissue state to colon cancer. Using molecular techniques, they looked in great detail at gene expression and fatty acid profiles of human colon samples, comparing cancerous tissues from people with colon cancer to two control groups: non-cancer tissue from other people without cancer; non-cancerous tissue from adjacent sections of colon from the cancer patients themselves. In other words, they had within-subject and between-subject non-cancer tissue samples they could compare to the cancer tissue.
In essence, they found that tumors exhibited an imbalance between pro-inflammatory and anti-inflammatory lipids. In simple terms, dietary ω-6 PUFAs like linoleic acid serves as precursors to lipid drivers of inflammation, such as prostaglandins (these are what non-steroidal anti-inflammatory drugs like aspirin counteract). ω-3 PUFAs, on the other hand, service as precursors for lipids that resolve the process of inflammation. In this way, ω-6 and ω-3 PUFAs have opposing effects on inflammation. In the colon cancer tissue, the balance of different pro- and anti-inflammatory lipids was out of whack, creating an inflammatory state.
Humans cannot endogenously produce ω-6 and ω-3 PUFAs. We obtain them from the diet. Both PUFA types compete for access to the same metabolic enzymes, which process both. As a result, imbalances in dietary intake of ω-6 and ω-3 PUFAs can result in imbalances in pro- and anti-inflammatory lipids in the body. In natural, pre-modern environment, ω-6 and ω-3 PUFAs were consumed in roughly equal proportion, so our bodies evolved to “expect” this. In the modern food environment, the dietary PUFA balance is heavily skewed towards ω-6s, favoring inflammation.
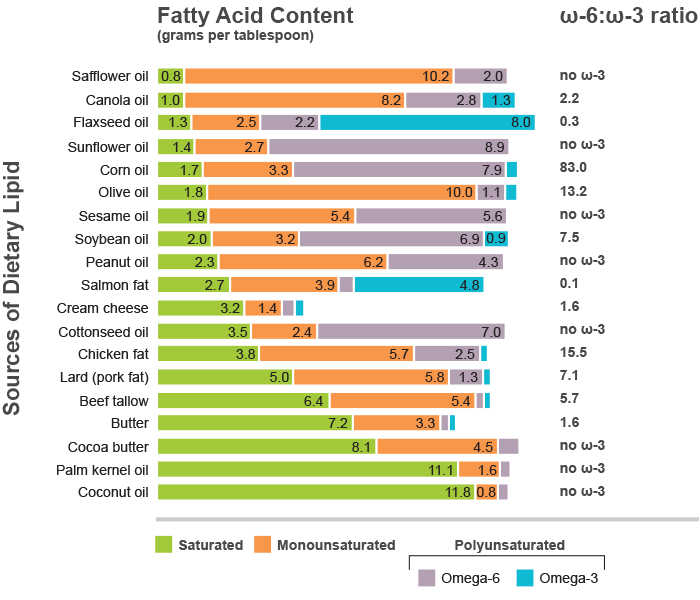
To give a sense of just how concentrated ω-6 PUFAs are in seed oils, consider this:

The basic biochemistry here has been known for many years, but Yeatman and Halade’s study directly measured, with high resolution, the specific lipids and gene transcripts needed to define exactly whether cancer tissue is indeed in a more inflammatory state than non-cancer tissue. This is indeed what they found, consistent with ideas from over a century ago (from Virchow) proposing that cancer resembles a chronically inflamed, poorly healing wound.

As discussed with the authors, the inflammatory state observed in colon cancer tissue was characterized by an imbalance in pro-inflammatory lipid products made from ω-6 PUFAs vs. pro-resolving lipids produced from ω-3 PUFAs.
Some commentators of this study tried to argue that it had nothing to do with seed oils or diet because the researchers did not directly measure or manipulate diet in people. While it’s true diet was not manipulated as a variable in the study, this is a silly and short-sighted view. The simple fact is that dietary PUFAs are the only source of the lipid mediators of inflammation in question, and seed oils are the dominant source of ω-6 PUFAs in people today.
Dr. Yeatman in his own words:
A concise summary integrating the findings from the lipidomics study and our conversation about that work:
Pro-Inflammatory Bias in Colon Cancer: Tumor tissue had elevated levels of pro-inflammatory lipid mediators like leukotrienes derived from arachidonic acid, a metabolite of ω-6 PUFAs.
Defective Lipid Class Switching: Colon cancer tumors showed a marked deficiency in lipid class switching, a process critical for resolving inflammation. This is due to low levels of pro-resolving mediators like lipoxins.
Dietary Omega-6 PUFAs and Seed Oils: The study highlights that ω-6 PUFAs are precursors to arachidonic acid, driving the production of pro-inflammatory mediators. The podcast emphasizes that the Western diet, high in ω-6 PUFAs, drives this imbalance.
Dietary Implications: The podcast clarifies that while the study didn’t directly prove seed oils cause colon cancer, the lipid dysregulation observed strongly suggests dietary ω-6 PUFAs as a primary source, given that these fatty acids must come from the diet.
Practical Recommendations: Yeatman and Halade recommend minimizing seed oil consumption by reading labels and opting for alternatives.
Industrial seed oils did not come on the scene until the early 1900s, and really started taking off in the mid-1900s. Advances in agricultural practices, together with government subsidies, produced a massive surplus in soybeans and other crops that could be used to make seed oils. Several decades ago, Procter & Gamble figured out how to market this surplus to consumers as “healthy vegetable oils,” paid off organizations like the American Heart Association, and the rest is history. Procter & Gamble proceeded to make bank from selling Crisco, which seems to have evolved into more business for proctologists today.
Learn more about the history of seed oils, agriculture, and human dietary patterns:
Article | Eating Fat Like Never Before
Article | The Cholesterol Cult & Heart Mafia: How the process of science evolves into The Science™ of public policy
When you understand the basic biology of fatty acid metabolism and our evolutionary history, together with the artificial demonization of animal fats that began in the 20th century, it is not at all surprising that chronic inflammation and metabolic dysfunction (including cancer) have risen in parallel with the explosion in ω-6 PUFA intake.
Why is colon cancer specifically on the rise in younger people? Think about it. People today are exposed to high levels of ω-6 PUFAs starting from before they are even born, because mom is probably consuming lots of ω-6 PUFAs. Your grandparents may have been exposed to plenty of ω-6 PUFAs over their lifetime, but they’re less likely to have started from such an early age. By constrast, babies today are commonly given formula that is little more than soybean oil masquerading as breast milk.
To put it another way: your odds of getting colon cancer depend on how much of your life you’ve spent funneling ω-6 PUFAs into one end of your tube and out the other. The average young person today has had high concentrations of ω-6 PUFAs sitting in their colon from the moment their colon developed.
The dietary fats we consume don’t just pass through or digestive tract—those fatty acids are digested, absorbed, and distributed throughout the body, incorporated into adipose tissue and lipid membranes of every cell in the body.
Other recent research has shown that ω-6 PUFAs like linoleic acid not only aggravate other forms of cancer, but are directly interacting with key metabolic pathways in the body that regulated growth generally. This suggests that we should expect high levels of ω-6 PUFA intake to be linked to many different forms of cancer and metabolic dysfunction. I happen to believe we’ve already been living through exactly that for the past several decades, and should expect more and more studies to explicitly demonstrate such linkages.
For now, let’s review another recent study linking ω-6 PUFAs to breast cancers via the mTOR pathway, a “master metabolic regulator” in the body…
Breast Cancer: Seed Oils, mTOR & Dysregulated Anabolic Growth
mTOR is one of the most famous and intensely studied pathways in all of biology, and for good reason. It is a master regulator of anabolic growth. In biochemistry, chemical reactions can be thought of in two broad classes: anabolic and catabolic. Anabolism means growth or building up tissues. Catabolism is the opposite, breaking stuff down. Every moment, your body is constantly building up and breaking down tissues and cellular structures based on its inputs and needs.
In M&M 229, Dr. John Blenis described mTOR as like a cellular “brain” that integrates many different signals and regulates growth-oriented (anabolic) processes in our cells. mTOR senses myriad signals, including levels of key nutrients like amino acids, sugar, and fats. If you want more detail, check out the podcast episodes below. For now, just think “growth” when you hear “mTOR”. When the mTOR pathway becomes more active, a cell will be in build mode.
Learn more about mTOR:
Podcast | Aging, mTOR, Sirtuins, Rapamycin, Metformin, the Truth of Resveratrol & Longevity Supplements, David Sinclair & Anti-Aging Myths | Matt Kaeberlein
There are many forms of healthy growth, ranging from the growth and differentiation of tissues that unfolds over the course of development, to the building of muscle tissue stimulated by resistance training and construction of new synapses in the brain as we learn. Anabolic processes that build up structures are highly regulated, and the mTOR pathway is a master orchestrator of anabolic growth.
Anabolic growth can also become dysregulated. When key regulators of growth become broken, this can lead to pathological forms of unregulated growth, which is what cancer is. Because cancer is a form of dysregulated cellular growth and mTOR is the master regulator of anabolic growth, we should expect the mTOR pathway to be involved in some way in pretty much all cancers.
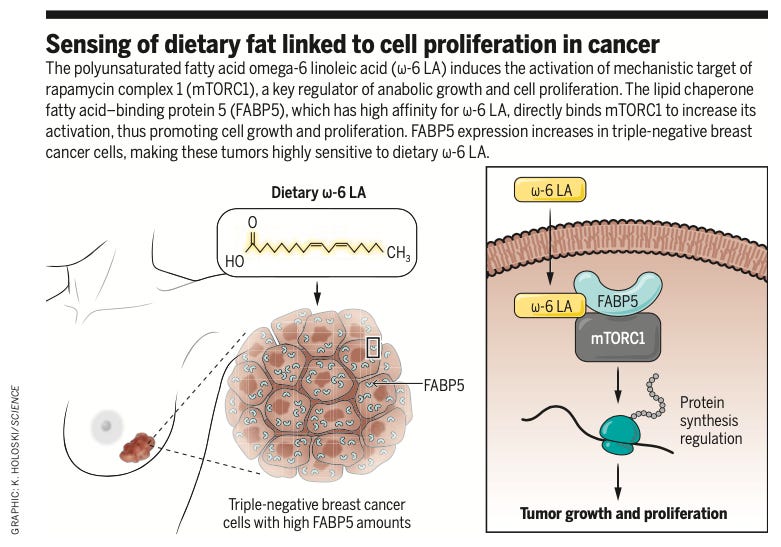
Sparing you the details, this new study demonstrated that linoleic acid, the principal ω-6 PUFA found in seed oils, has a direct effect on mTOR and fuels the growth of “triple-negative” breast cancer. This is not the most common form of breast cancer, but is particularly deadly version that happens to be rising in younger age cohorts.
This story has three main players:
Linoleic acid: The most abundant dietary ω-6 PUFA. By some estimates, the average American is getting 20% or more of their daily calories from this one molecule. This intake pattern is without precendent in human history.
mTORC1: One of the two main mTOR complexes. “Complex” here just means that a cluster of molecules that come together and act one functional unit in a cell. They often work by acting as transcription factors that go into the nucleus and regulate gene expression. mTORC1 in particular is exquisitely sensitive to levels of growth factors and nutrients.
FABP5: A “lipid chaperone protein.” It’s function is to help shuttle fatty acids from the outside of cells to the inside. In this case, FABP5 is how linoleic acid gets into cells, enabling it to stimulate mTOR and fuel triple-negative breast cancer.
They did a variety of in vitro experiments to work out how linoleic acid was plugging into the mTOR pathway. As it turns out, triple-negative breast cancer cells (but not other cells) express high levels of FABP5, the lipid chaperone protein needed for linoleic acid to get into cells. The aggressive growth of these cancer cells is fueled by linoleic acid because they’re good at sucking it up from their surrounding due to high levels of FABP5 expression.
Over-expression of FABP5 effectively makes cells more sensitive to linoleic acid-fueled growth. If triple-negative breast cancer cells didn’t express so much of this lipid chaperone, they wouldn’t be able to import as much linoleic acid and therefore couldn’t grow as aggressively.
In addition to working out the biochemical details at the level of cells, they did experiments in rodents and examined human triple-negative breast cancer tissue. In rodents that already had triple-negative breast cancer, boosting the intake of dietary linoleic acid aggravated the cancer. Tumor growth was promoted in animals fed a diet enriched in safflower oil. While safflower oil contains linoleic acid and was sufficient to promote tumor growth in these animals experiments, it’s worth noting that there are plenty of other seed oils with even higher linoleic acid levels (see below).
When they examined tissue samples from human patients with triple-negative breast cancer, they observed elevated levels of both linoleic acid and FABP5, indicating that things are likely working in a similar fashion in people.
Experiments with the ω-3 PUFA α-linolenic acid (ALA) showed distinct effects. ALA did not activate mTOR or aggravate tumor growth in animals. If you study PUFA metabolism in detail, this won’t be surprising. ω-6 and ω-3 PUFAs are widely observed to have distinct and even opposite biological effects. The distinct effects of linoleic acid (ω-6) and ALA (ω-3) seen in this study add more evidence on the importance of ω-6:ω-3 balance for health, and why the heavily ω-6-skewed diet of modern people is a problem.
Remember: the American Heart Association and the majority of practicing physicians today will still tell you ω-6 PUFAs like linoleic acid are “heart healthy,” for no other reason than that they lower LDL-cholesterol levels.
As with most things, FABP5 expression probably varies naturally between cell types and between individual people is. I’m not sure we know how much variability exists in FABP5 across human populations, but to the extent there variability, this would mean that individuals with higher FABP5 levels would be more sensitive to linoleic acid-driven mTOR signaling and growth. In any case, we already know that humans display a high level of genetic diversity in key enzymes responsible for the metabolism of ω-6 PUFAs. I strongly suspect this type of metabolic variation contributes to variation in “PUFA tolerance,” meaning that some people will be more prone to ailments rooted in PUFA-driven inflammation, including cancer.
Learn more about human metabolic diversity and evolution:
Article | What Did Humans Evolve to Eat?
Podcast | Evolution & Genetics of Human Diet, Metabolism, Disease Risk, Skin Color and Origins of Modern Europeans | Eske Willerslev
Human Metabolic Variability & “Nutrient Sensitivity”
Humans are less genetically diverse than many other animal species, but there is still plenty of meaningful genetic diversity between human populations. The genes encoding various metabolic enzymes often display among the highest levels of diversity in the human genome, and there’s growing evidence that genes involved in nutrient metabolism have undergone strong natural selection since the dawn of large-scale sedentary agriculture a little over 10,000 year ago.
Key enzymes involved in fatty acid metabolism are the FADS genes, which encode fatty acid desaturase enzymes. One important role FADS enzymes play is converting dietary ω-6 PUFAs like linoleic acid into arachidonic acid. From there, arachidonic acid can be converted into inflammatory lipid mediators of inflammation, such as prostaglandins, which we discussed above in the colon cancer story.
The key thing I want to convey here is that variation among people in their FADS enzymes controls how readily dietary ω-6 PUFAs are converted into downstream products. This variation means that two different people can have the exact same pattern of dietary PUFA intake, but experience different health outcomes. Even if every aspect of their lives were identical, apart from their FADS enzymes, their bodies would metabolize dietary PUFAs in a different pattern. One person might convert linoleic acid to arachidonic acid at a higher rate, providing a larger substrate that could be converted into pro-inflammatory lipids. Another person might convert less of their dietary linoleic acid into arachidonic acid, leaving more linoleic acid around to potentially get inside of cells and stimulate mTOR, depending on expression of the FABP5 lipid chaperone we discussed in the breast cancer story. FABP5 expression itself probably varies across tissues and individuals (to some unknown extent).
As shown in studies like this, genes involved in fatty acid metabolism vary widely across human populations. This likely impacts our sensitivity to dietary PUFAs, with implications for chronic disease susceptibility, energy homeostasis, immunity, and brain function.
Multiple studies, such as this one, have mapped out human genetic diversity in fatty acid metabolism. Here’s a geographic map of how this diversity maps out across human lineages:
The point is that patterns of dietary fat intake are not the only source of variation in health outcomes when it comes to dietary fats like PUFAs. Everyone has a different metabolism and will be affected by a given pattern of nutrient intake differently from other people. Some of these metabolic differences come from differences in non-genetic factors, such as life history patterns related to nutrient intake. But there are also intrinsic differences between people, such as differences in enzyme efficiency or selectivity stemming from genetic variation.
This type of metabolic variation is rarely accounted for in clinical studies looking at the effects of diet on human health. This is a major reason why the results of clinical studies can produce conflicting results and should not be taken at face-value without close scrutiny of the patient populations and how the data were collected and analyzed.
I discussed these issues more in this article, which goes into more detail on how toxic chemicals are produced from lipid peroxidation of PUFAs, which is another reason why we expect dietary PUFAs to be linked to chronic inflammatory diseases generally, not just specific forms of cancer.

People's background PUFA intake, fat tissue composition, and fatty acid metabolism vary greatly. The genes encoding fatty acid desaturases, which metabolize PUFAs, are also highly variable. There is evidence that, following the transition to agriculture, there was strong natural selection on fatty acid metabolism. Sedentary farming drastically changed the food environment people lived in, including their ω-6 PUFA intake. The specific genetic variants you have influence things like your LDL cholesterol levels, likely playing a role in the extent to which ω-6 PUFAs affect your biology. However, clinical studies pretty much never account for these critical differences between individuals.
Metabolic diversity among people needs to be accounted for in the design of clinical studies and kept in mind when we interpret results. It’s also important to consider metabolic diversity when thinking about your own diet and health. The specific genetic lineage we belong to and our unique life history patterns, including past exposures to nutrients and metabolism-disruptors, will shape how our bodies respond to what we put in them. Two different people can experience vastly different outcomes on the same diet.
While some people might be more “tolerant” to ω-6 PUFAs and therefore less sensitive to their negative effects, that does not mean that high levels of ω-6 PUFA intake are “good” for some people. It really just means that you may be able to tolerate a higher dose before stuff starts breaking in noticeable ways. No matter who you are, no one is “meant” to get 20% or more of their calories from linoleic acid, or consume ω-6 PUFAs in great excess of ω-3 PUFAs. The underlying biology tells us what to expect if we do: inflammation and chronic disease.