Seed Oils & Body Fat: Endocannabinoid Regulation of Food Intake & Energy Storage
The endocannabinoid system coordinates metabolic changes across the brain and body to favor energy storage. Dietary ω-6 PUFAs boost endogenous cannabinoids.
Not medical advice.
M&M written content is human-generated, independently researched, and filled with links to resources to deepen your learning. Click here to see all the ways you can support my work.
This post is one of several exploring the relationship between dietary ω-6 polyunsaturated fats and obesity, fatty liver disease, and adiposity generally. Each post explores one or more of the Overall Points listed below.
Related articles:
Seed Oils & Body Fat: When did modern obesity rates begin rising?
Seed Oils & Body Fat: ω-6 PUFAs cause obesity & fatty liver
Overall Points (across articles):
Seed oil consumption has steadily risen since the 1800s, correlating with rises and falls in obesity better than carbohydrates or saturated fat.
In animals, high linoleic acid diets drive weight gain more than alternative diets, even when alternatives have less saturated fat or more sugar.
In animals, boosting dietary linoleic acid content from ~1% to ~8% of energy is sufficient to drive weight gain, adiposity, and fatty liver. Today, the average American gets >10% of calories from linoleic acid.
Animals genetically engineered to convert ω-6 PUFAs into ω-3 PUFAs are resistant to obesity and other forms of metabolic dysfunction.
The obesogenic effects of seed oils are tied to the endocannabinoid system, which is functionally conserved across species (including humans).
The evolutionary purpose of this biology is to promote energy intake and storage in times of plenty, resulting in survival during future periods of scarcity.
Large RCTs with proper tissue measurements and dietary control are lacking. Such trials would need to last for several years to detect the obesogenic effects observed in preclinical studies.
Observational human studies that carefully measure tissue levels of ω-6 and ω-3 PUFAs over time are rare. Those that exist show a positive association between obesity and ω-6 PUFAs.
Weight loss drugs past and present work downstream of ω-6 PUFAs, along the pathway linking them to endocannabinoid regulation of reward, whole-body metabolism, and feeding behavior.
Metabolism & the Endocannabinoid System: Brief overview
The endogenous cannabinoid system (ECS) plays a critical role in maintaining homeostasis across tissues. The ECS is perhaps most well-known for its actions with the nervous system, largely due to the psychoactive effects of the exogenous cannabinoid THC. But endogenous cannabinoids and their receptors are located throughout the body, where they regulate the function of diverse cells in organs throughout the body.
There are two major endogenous cannabinoids, anandamide and 2-AG, plus two major cannabinoid receptors, CB1 and CB2 receptors. Despite a relative bias for CB1 receptors in the central nervous system and CB2 receptors elsewhere, both cannabinoid receptors are located all over. Through their action on CB1 receptors in the brain, endocannabinoids are well-known to regulate appetite and feeding behavior.
In general, higher endocannabinoid levels (“endocannabinoid tone”) lead to more CB1 receptor activation. In the brain, this alters sensory perception and behavioral motivation to favor energy acquisition; in other tissues, it leads to metabolic changes favoring energy storage.
As overviewed in this article, CB1 activation results in the following metabolic effects across organ and tissue systems, across species:

A somewhat underappreciated aspect of endocannabinoid biology is that endogenous cannabinoids are derivatives of dietary ω-6 polyunsaturated fatty acids (PUFAs), and that dietary PUFA intake can alter endocannabinoid tone, the general levels of endocannabinoids in the brain and body.
Learn more about the fundamentals of endocannabinoid biology:
Article | Metabolic Effects of Cannabinoids
Podcast | Endocannabinoids, Stress, Exercise, Cortisol, Anxiety, Cannabis & Effects of Marijuana on Brain Development | Matthew Hill
The Seed Oil-Endogenous Cannabinoid Connection
The lipid biology here gets complex, fast. The essential point to grasp for now is that dietary linoleic acid, the main ω-6 PUFA in seed oils, faces different possible fates after being consumed:
Metabolized for ATP production through mitochondrial β-oxidation.
Used as a structural component (e.g. cell and mitochondrial membranes).
Enzymatically transformed into other lipids, which can serve some function.
When linoleic acid is incorporated into membranes as a structural component, it may undergo lipid peroxidation by Reactive Oxygen Species (ROS)—inadvertently “set on fire,” producing cell toxins like 4-HNE that damage cellular structures and can even trigger cell death. This is a big part of oxidative stress and an inevitable consequence of oxidative metabolism. Cells are never “trying” to do this—in the presence of oxygen and heat, it will happen at some rate.

A second possible fate of dietary PUFAs is transformation by enzymes into other lipids. Our cells do this “on purpose.” Fatty acid desaturase (FADS) enzymes turn linoleic acid into arachidonic acid, another ω-6 PUFA. From there, additional transformations can turn arachidonic acid into a variety of effector molecules, such as inflammatory prostaglandins or endocannabinoids.
This diagram will give you a taste for how dietary PUFAs get transformed to endocannabinoids in the brain, but it happens in other tissues as well.
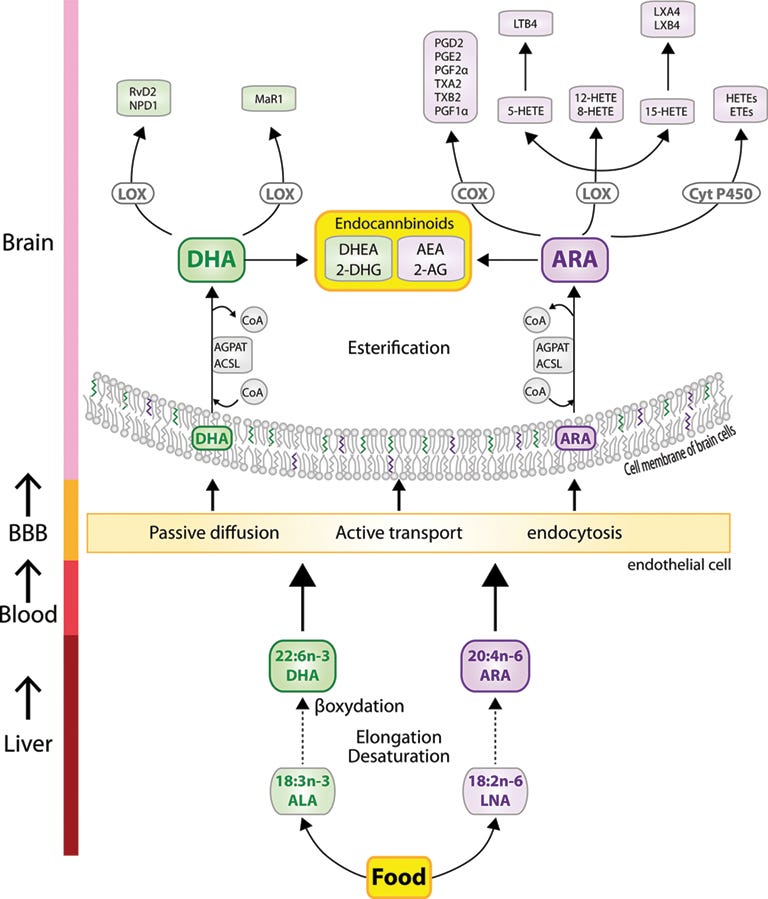
FADS genetics constrain the rate at which linoleic acid is converted into arachidonic acid, influencing the size of the arachidonic acid pool available to produce downstream effectors like endocannabinoids. As a rule, higher intake of dietary PUFAs will increase the pool of ω-6 PUFAs from which endocannabinoids are produced. This influences “endocannabinoid tone,” the general level of endogenous cannabinoids your body produces at any given time.
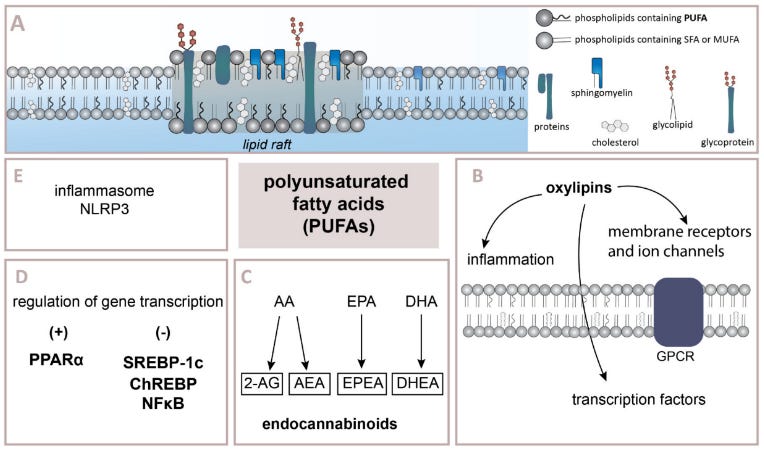
Dietary PUFAs, Endocannabinoid Tone & Food Intake.
This general tendency to keep in mind:
Increased dietary ω-6 PUFA intake elevates endocannabinoid tone.
Elevated endocannabinoid tone leads to more CB1 receptor activation in the brain and body (“CB1 signaling”).
Increased CB1 signaling promotes metabolic changes favoring food intake and energy storage.
Endocannabinoid tone modulates energy homeostasis, appetite, and reward-driven eating by acting in both the brain and peripheral organs (liver, adipose tissue, etc). Dietary PUFA intake influences endocannabinoid tone. High linoleic acid intake increases tissue levels of arachidonic acid, the precursor for anandamide and 2-AG, leading to elevated endocannabinoid levels and enhanced CB1 signaling.
Similar to their function opposition in other regards, such as inflammation, ω-6 and ω-3 tend to have opposing effects here: oral ω-6 PUFA rapidly increases endocannabinoid levels in the gut, which then signals to the brain to promote further fat intake. Diets rich in ω-3 fatty acids can counteract this effect by reducing the pool of arachidonic acid and normalizing endocannabinoid tone.
In this article, we saw that seed oils drove obesity and fatty liver in animals. What we didn’t discuss was how it happened: dietary linoleic acid elevated tissue arachidonic acid and endocannabinoids in multiple tissues, including the brain, liver, and red blood cells. The elevation of tissue endocannabinoids by dietary linoleic acid has been observed multiple times, in species as diverse as mice and fish.
The effects of elevated dietary linoleic acid in animals include increased weight gain, fatty liver, adiposity, and inflammation. In small human studies of overweight or obese individuals, a high linoleic acid diet seems to promote excess energy intake, although such studies do not last nearly as long as would be needed to see the types of obesogenic effects observed in rodents over many weeks.
The endocannabinoid system (ECS) and its core components are evolutionarily conserved across animal species. Exogenous cannabinoids like THC and elevated levels of endogenous cannabinoids produce similar overall effects in rodents and humans, such as stimulation of appetite. This is why CB1 receptor activation results in similar effects in both mice and humans:

Learn more about the underlying research in this short paper, or my conversation with the author:
Paper | Metabolic Messengers: endocannabinoids
Podcast | Cannabinoid System: Metabolism, Evolution & Energy Storage | Giovanni Marsicano
Evolutionary conservation explains why both endogenous and exogenous cannabinoids have similar effects on feeding behavior and metabolism across species. It also explains why randomized controlled trials in obese patients observe signs of endocannabinoid dysregulation in adipose tissue, with the expression of endocannabinoid proteins sensitive by diet.
That’s the “how” and the “what.” But what about why?
Why is it is animal biology setup this way? How would a body-wide, diet-sensitive regulatory system like this actually favor survival and reproduction?
The Core Evolutionary Function of CB1 Receptor Signaling
Keep reading with a 7-day free trial
Subscribe to Mind & Matter to keep reading this post and get 7 days of free access to the full post archives.



