Dietary Fat, Sunscreen & Skin Photosensitivity: Can Seed Oils Promote Sunburn?
The body's sensitivity to the world depends on what you feed it.
Not medical advice
Podcast episodes that informed this article:
M&M 233: Feel the Burn: Seed Oils, Memes & Oxidative Stress | Brian Kerley
M&M 146: Photobiology, Sunlight, Firelight, Incandescent Bulbs vs. LEDs, Mitochondria, Melatonin, Sunscreen & the Optics of the Body | Scott Zimmerman
M&M 221: Regenerative Energy & the Light Inside You | Jack Kruse
Basic Biology of Oxidative Stress & Sunburns
Reactive oxygen species (ROS) are an inevitable consequence of oxygen-based metabolism. Within our cells, mitochondria are the predominant site of oxygen-based energy and ROS production.
Mitochondria in our cells are always producing ROS at some rate, as a natural consequence of oxygen-based metabolism. Evolution has equipped cells with antioxidant systems that can sense ROS production and quench ROS before they do real damage. As long as ROS production does not exceed antioxidant capacity, there’s not much to worry about.

When ROS production exceeds antioxidant capacity, nucleic acids, proteins, or lipids within our cells take on oxidative damage. In short: ROS break stuff. Minor breaks can be repaired. Major damage requires more substantial repairs, with higher energy cost. Dropped a cup of coffee in the kitchen? Annoying, but no big deal—two minutes to clean up. Threw an all-night rager, and people got wild? The next day, your finite energy budget is spent scrubbing floors and spackling drywall, instead of relaxing. With persistent negligence, things can get worse—the kitchen catches on fire, or the house burns down. Do you have insurance? How much does it cover?
(Side note: there are also reactive Nitrogen Species, RONS. We will simply talk about ROS here, but similar ideas apply: highly reactive molecules break stuff if not properly contained.)
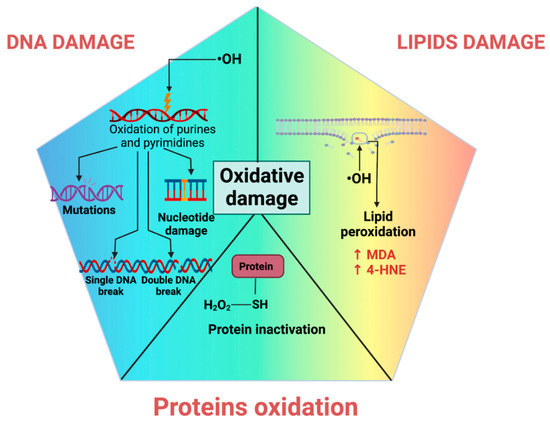
All cells have a finite energy budget, and may or may not be well-insured depending on cell type. The greater the level of oxidative stress cells experience, or the lesser the ability to deal with it, the more likely the damage is to exceed the energetic needs of repair and housekeeping mechanisms. And if the repair mechanisms themselves become damaged, what then? This is part of what underlies aging and senescence—cells are functionally compromised and either can’t or won’t rejuvenate. At some point, core mechanisms of repair and rejuvenation are themselves broken. Grandpa has gone senile.
ROS are not all bad, though. Cells use them to do useful work. Consider how people use fire in their homes: to cook food, heat the home, and so forth. When done on purpose, for some utilitarian reason, fire is contained and regulated—you control where and when flames are ignited, with precautionary systems in place (fire extinguisher, etc.) in the event the flames burn out-of-control. Our cells utilize a certain level of burning to do useful work, with systems that can contain the fire—up to a point, that is.
Endogenously produced ROS are not the only source of flames. Small fires can also be ignited by external physical stimuli, such as UV light, toxic chemicals, etc. The surfaces of our body—both the outer surface (skin), and inner surface (gut lining)—are exposed to these things the most. Naturally, they have evolved to deal with such stressors, up to a point. There’s a reason your skin and gut cells regenerate quickly when damaged: they occupy high-risk real estate within the organism.
Our focus here will be on how the skin deals with light stress, and the ways in which materials that we ingest or apply to the skin surface influences skin’s ability to deal with it.
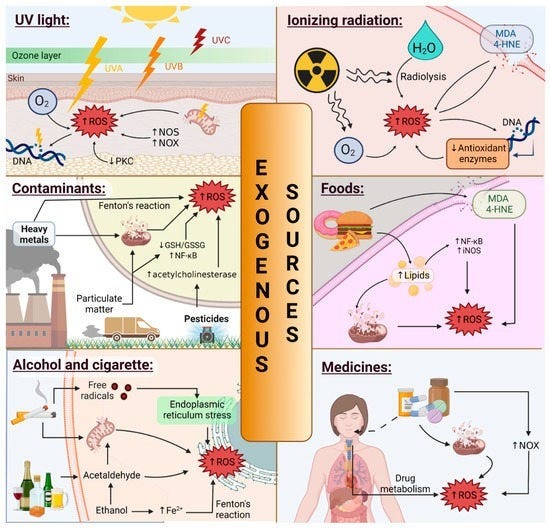
Skin cells have some natural capacity to absorb and utilize light, as well as natural antioxidant capacity to absorb and utilize some level of ROS. The paler your skin is, the lower its capacity to absorb UV, and the more readily oxidative stress is induced. If this happens enough, it triggers an inflammatory response: pro-inflammatory signals are secreted to attract other cells that will clean up debris and tissue damage. When a building is demolished, you first need to clear our the construction site before new construction can be properly executed. This comes with vasodilation (redness), swelling, and so forth.
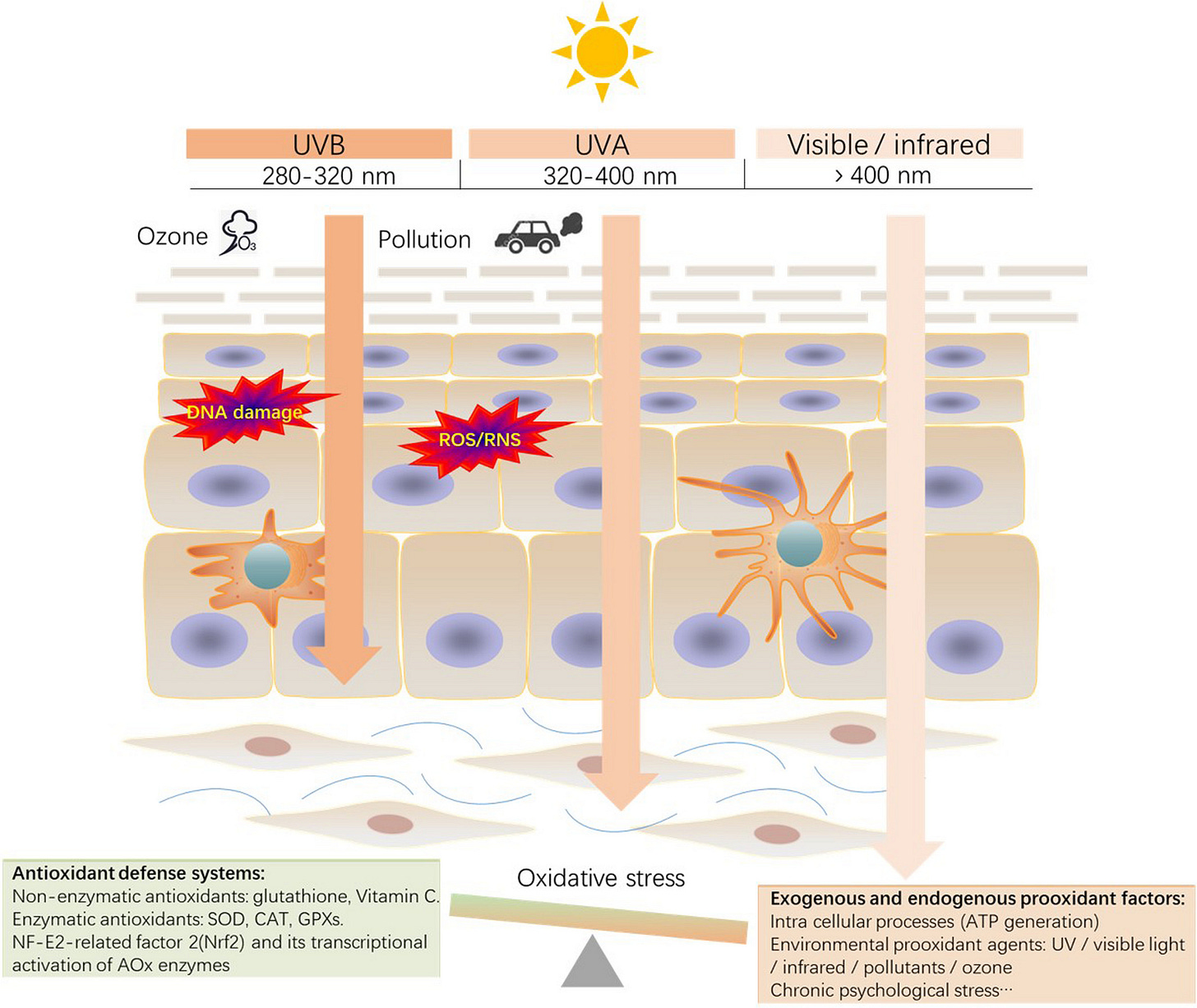
To put it more concisely: sunburn is a manifestation of oxidative stress in your dermal tissue. The redness, burning sensations, and skin peeling are symptoms arising from “too much” oxidative stress, which eventually drives cell death and inflammation. Luckily, skin cells have “insurance.” The skin peels, but dead cells are quickly replaced. Inflammation makes way for rejuvenation. Melanogenesis (increased melanin production) can also be stimulated, a light-absorbing pigment that acts as a natural sunscreen, enabling you to tolerate more sunlight exposure in the future.
Learn more about mitochondria & oxidative stress:
M&M 70: Mitochondria, Aging, Cellular Energy, Metabolism, Gray Hair Reversal & Brain-Body Communication | Martin Picard
M&M 220: Cell Death, Oxidative Stress, PUFAs & Antioxidants | Pamela Maher
Ultraviolet, Non-UV Light & Oxidative Stress
UV light is a major driver of sunburn, but other wavelengths also contribute to oxidative stress. Longer wavelengths of light, including visible light, penetrate our skin more deeply than UV. If you cover your skin in commercial sunscreens, they block UV wavelengths, enabling you to stay in direct sunlight for longer without your senses tell you to seek shade. And as long as you do, longer non-UV wavelengths penetrate your skin.
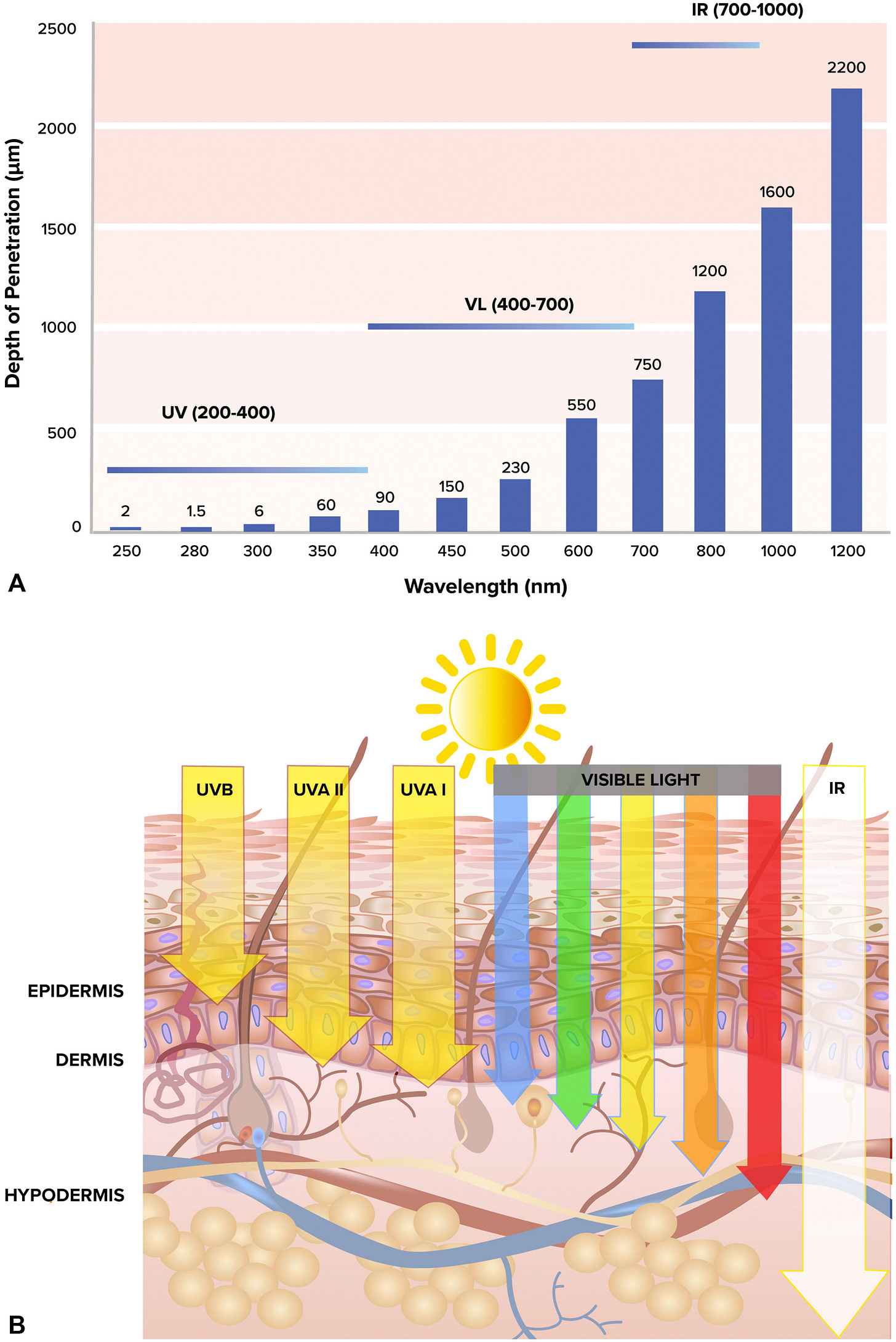
Obviously, the more time you spend under direct UV, the more likely your skin is to eventually sunburn. Everyone has a different tolerance level based on things like melanin content, but oxidative stress cannot be completely avoided. No one is totally immune from the sun, despite large differences in tolerance levels.
To repeat: nobody can withstand an arbitrary amount of sunlight, especially intense sunlight, without sustaining tissue damage. There is variation and plasticity in tolerance levels, however, allowing skin to adapt to light conditions, up to a point. The pattern and frequency of sunlight exposures you have informs biological adaptations, such as melanin production, that alter your skins’ propensity to sunburn.
Skin’s propensity for sunburn can change as a function of how much pigment it produces. Does it also change as a function of the fatty acid composition of your lipids?
Let’s briefly review how dietary PUFAs related to oxidative stress, which will help us think about how they might tie into dermal photosensitivity.
Learn more about skin and photobiology:
M&M 104: Benefits & Risks of UV Radiation & Sunlight, Skin Health, Vitamin D, Nitric Oxide, Evolution of Skin Color | Richard Weller
M&M 146: Photobiology, Sunlight, Firelight, Incandescent Bulbs vs. LEDs, Mitochondria, Melatonin, Sunscreen & the Optics of the Body | Scott Zimmerman
Dietary PUFAs, Oxidative Stress & Skin Photosensitivity
Like other cells, skin cells have a phospholipid bilayer membrane filled with fatty acids. The fatty acids vary in length and degree of saturation. In any given tissue there is some distribution of fatty acids in lipid membranes. Diet is an important factor influencing the specific fatty acid profile that membranes have.
Polyunsaturated fatty acids (PUFAs) are inherently easier to oxidize than monounsaturated or saturated fatty acids, which makes them more sensitive to ROS. More carbon-carbon double bonds means higher ROS sensitivity. The higher the PUFA concentration in your cell membranes, the more likely they are to “light on fire” if ROS levels exceed antioxidant capacity and start reacting with membrane lipids.This can ultimately drive forms of cell death (e.g. ferroptosis).
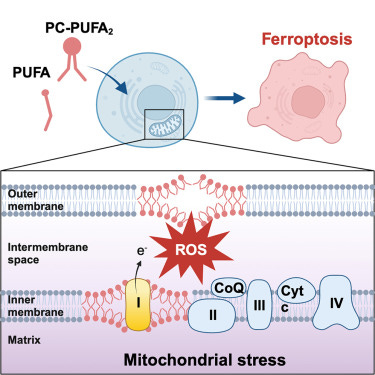
The idea is that higher PUFA concentrations in your skin cell membranes make you more sensitive to sunburn. If there’s less dry kindling to burn, your cells will be able to tolerate more “small fires” ignited by UV light before the house burns down (skin cells die). Eventually, if skin cells are exposed to enough UV light, they’re just going to burn no matter how high your tolerance level is. The question is how much they can tolerate.
That’s the idea, anyway. But is it true in practice? And significant enough for your sunburn propensity to noticeable change with a dietary shift?
Does changing the fat you put into your body actually affect your sensitivity and tolerance to sunburn? Some say that it does and that by minimizing seed oil consumption you decrease your sensitivity to sunburn and therefore tolerate more sun exposure before burning. The less dry kindling in your membranes, the more energy it takes to light the house on fire. Others find this idea preposterous.
Before directly examining the question of whether dietary PUFAs from seed oils can affect dermal photosensitivity and sunburn potential, let’s look at how other substances impact skin photosensitivity, either when put on the skin surface or ingested. This will give us a sense for what’s possible and the ways in which our skin’s photosensitivity can be changed. We will consider:
Natural “sunscreens” like melanin in relation to tanning and sunburn.
Topical commercial sunscreens in relation to light absorption and oxidative stress.
Prescription drugs and food components that change skin photosensitivity.
Effects of topical cholesterol and PUFAs on skin photosensitivity.
With an understanding how all these things relate to light absorption, oxidative stress, and dermal photosensitivity, we will be in a better position to reason about whether changing your dietary fat intake is likely to impact your skin’s light sensitivity.
Let’s start with the basics on skin plasticity and melanin production to help us think about how and why our skin’s photosensitivity can change in response to the physical environment.
Learn more about PUFAs, lipid peroxidation, and cell death:
Podcast | Cell Death, Oxidative Stress, PUFAs & Antioxidants | Pamela Maher
Article | PUFAs & The Palisades: Lipid Peroxidation, Oxidative Stress & Cell Death
Basics of Melanin Biology & Skin Plasticity
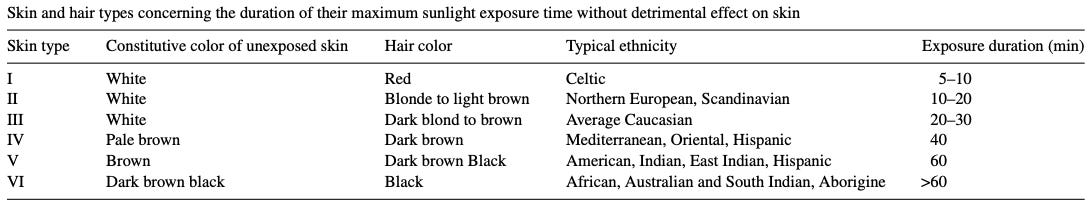
The amount of melanin in one’s skin and hair sets limits on how much direct sun exposure of a given intensity one can tolerate before detrimental outcomes manifest. Approximations for the major skin and hair color types of humans:
In simplified terms, the basic way compounds like melanin or those found in chemical sunscreens work is that, based on chemical structure, they are able to absorb photons within a certain range of wavelengths (colors). Notice that the structure of melanin contains conjugated rings with multiple double-bonds:
Chemical structures like this have electrons that can be excited by photons, enabling the molecule to capture light. The key thing here is simply that the melanin concentration of skin effectively determines “UV tolerance,” which is basically how much UV you can handle before oxidative damage begins to “overflow” beyond what antioxidant systems can handle. The more melanin in your skin, the less oxidative stress per unit time in sunlight, because some of that UV will be absorbed by melanin instead of causing direct damage or generating reactive oxygen species (ROS).
(Note: Sunlight can also stimulate antioxidant production, not just oxidative stress. The pattern and intensity of wavelengths matters. For example, red and near-infrared light can stimulate mitochondria function the body, as these wavelengths penetrate beyond the skin. This can influence things like melatonin production, which is a potent natural antioxidant. See M&M 146.)
Melanin in human skin primarily exists as eumelanin and pheomelanin, with eumelanin being the dominant form in most people. Each is synthesized from amino acid precursors:

Eumelanin: A brown-black pigment, highly effective at absorbing UV radiation, providing photoprotection. It is the primary contributor to darker skin tones.
Pheomelanin: A reddish-yellow pigment, less effective at UV absorption, found in higher proportions in lighter skin and red hair. It may contribute to UV-induced damage due to its photochemical properties.
Melanin is synthesized by melanocytes in the epidermis and stored in organelles called melanosomes, which are transferred to surrounding keratinocytes. The size, number, and distribution of melanosomes influence skin pigmentation.
A diagram to help you appreciate the complexity of pathways linking UV exposure and DNA damage to ROS production and melanin synthesis.

The melanin absorption spectrum is broad, spanning UV, visible, and near-infrared, with stronger absorption at shorter wavelengths. By absorbing external UV light, melanin can protect skin by:
Dissipating UV energy as heat, reducing DNA damage.
Scavenging reactive oxygen species (ROS) generated by UV.
Eumelanin is more efficient at photoprotection than pheomelanin. Pheomelanin can actually elevate ROS production, triggering things like lipid peroxidation or DNA damage. This why red-headed individuals are very sensitive to light-induced oxidative skin damage, as they produce higher levels of pheomelanin compared to eumelanin.
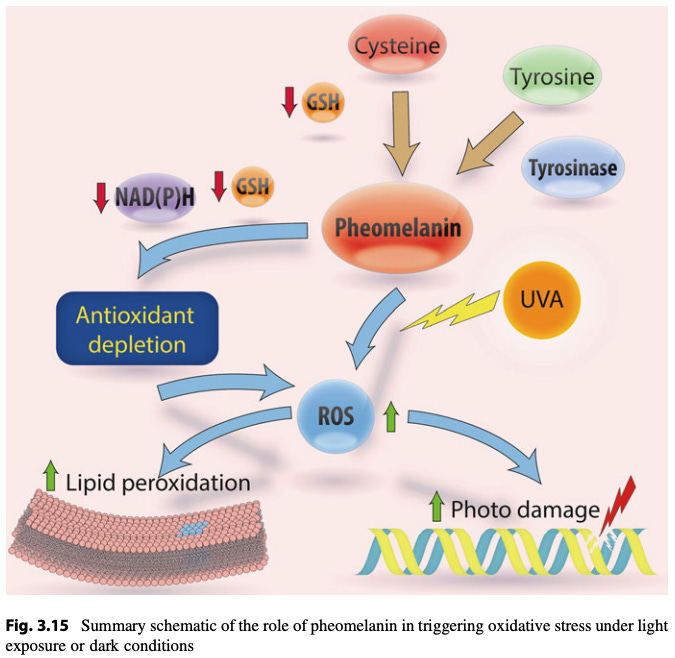
Getting intermittent sunlight exposure such that eumelanin is boosted results in greater sun tolerance. Increased melanin levels mean that more UV will be absorbed and dissipated rather than doing direct damage. Melanin also scavenges ROS, helping prevent them from doing damage or triggering lipid peroxidation in cell membranes which would generate more toxic molecules. As a result, a given amount of direct sun exposure produces less overall oxidative stress than it would for someone with lower eumelanin levels (all other things being equal).
That much should be intuitive. Now ask yourself: what other biological “knobs” can be changed in our cells to decrease light-induced oxidative stress, other than boosting melanin levels? In other words, what else would lower the overal oxidative stress burden of a light-exposed cell?
(Hint: if there’s lower PUFA density in cell membranes, fewer toxins can be produced from lipid peroxidation—less “flammable material” will be lying around, so ROS will have lower odds of setting the house on fire).
Skin’s ability to change melanin levels in response to sunlight exposure enables our sunlight sensitivity, including sunburn propensity, to change in response to environmental conditions. Melanin is basically a natural sunscreen produced within the skin. In comparison, how to artificial sunscreens applied to the skin surface work?
Artificial Sunscreens: Do they Enable More Oxidative Stress in Skin?
Artificial sunscreens come in two general varieties. “Chemical sunscreens” contain molecules that absorb UV photons; “physical sunscreens” contain minerals that reflect photons. Many natural biomolecules can absorb photons as well. The most famous in this regard is melanin (discussed above).
Light-absorbing molecules often contain multiple aromatic ring structures. For our purposes here, just know that light absorption depends on chemical structure, and aromatic ring structures tend to be good at this. A diagram showing how oxybenzone, a common commercial sunscreen molecule, absorbs UV light:

Here’s the thing: most popular chemical sunscreens only block a narrow band of sunlight from your skin, mostly just UV. At first blush, that might not seem problematic. After all, UV light can be mutagenic to DNA, induce oxidative stress, and eventually result in sunburn. While true, many overlook that non-UV light can also induce oxidative stress.
In essence, commercial sunscreens help prevent you from UV-induced sunburns, allowing you to stay in strong, direct sunlight for longer. In doing so, your skin is exposed to more sunlight overall, including oxidative stress-inducing wavelengths that your sunscreen does not block.
End result: it’s possible to generate as much or more overall oxidative stress while wearing sunscreen and staying in direct sunlight for long periods, despite blocking UV photons. I discussed this with a photobiology researcher on M&M 146:
Scott Zimmerman: In [classic] work by Zastrow, who was in the cosmetics industry, he measured the amount of free radical generation as a function of wavelength throughout the visible spectrum and into the near-infrared. Some modern sunscreen is mainly [blocking] UVA, UVB, and UVC, and then there’s a cutoff.
What Zastrow showed was that, if you take skin and measure the free radicals, with exposure to the UV portion of the light spectrum, you get X [some amount of free radical production]. If you then take skin, block UV but exposing it to visible light, you get X again. There’s equal amounts of free radicals being generated from the visible light.
So what ends up happening when people use sunscreen? [It mostly blocks UV light,] the portion that actually gives you sunburn, which would tell [your senses], “Hey dummy, it’s time to get out of the sun.” Well, now you block that and you stay outside longer, increasing your exposure to the visible portion of the spectrum.
NJ: And that’s also generating oxidative stress. So is what you’re saying that most sunscreens actually make it easier for people to expose themselves to more overall oxidative stress because they can stay out in the sun for longer?
SZ: Yes.
Note: The reason many commercial sunscreens don’t block out a wider band of light is that doing so would cause your skin to be colored when you apply it. Covering yourself in mud works, for example, but nobody wants to do that. Even mineral sunscreens that leave a visible white residue often turn people off. Hence, the multibillion-dollar commercial sunscreen industry is geared towards chemical sunscreens that block narrow bands of sunlight and aren’t visible to the eye, driven by consumer spending patterns.
Here is an excerpt from some of the research done by Zastrow et al., referred to above:
In the absence of sunburn as an unmistakable warning, applicants use to over-exposing themselves to solar radiation, sometimes up to 10 times longer. As the standard commercial sunscreens, however, provide no protection in the VIS/IR spectral region, the free radicals being formed in this spectral region can easily overcome the critical radical concentration (FRTV). Thus, it is not surprising that skin cancer incidence is still rising although the use of sunscreens exhibiting high SPF values has become increasingly popular.
Knowing this, the following fact, often overlooked and usually surprising to people, should make sense: melanoma, the most deadly form of skin cancer, is not actually linked to sunlight exposure. Many, including dermatologists, presume that it is. The evidence is thin. The more common and far less deadly form of skin cancer, basal cell carcinoma, is correlated with UV/sunlight exposure, but you might be surprised at what the data actually shows for other skin cancers, especially for melanoma. A short excerpt from this excellent article:
What’s critically important to understand about melanoma is that while it’s widely considered to be linked to sunlight exposure—it’s not. For example:
Patients with solar elastosis, a sign of sun exposure, were 60% less likely to die from melanoma.
Melanoma predominantly occurs in areas of the body with minimal sunlight exposure, unlike SCC and BCC, which are linked to sun-exposed regions.
Outdoor workers, despite significantly higher UV exposure, have lower rates of melanoma compared to indoor workers.
Many sunscreens contain toxic carcinogens (to the point Hawaii banned them to protect coral reefs). Conversely, existing research indicates widespread sunscreen use has not reduced skin cancer rates.
•A mouse study designed to study malignant melanoma found mice kept under simulated daylight develop tumors at a slower and diminished rate compared to those under cool white fluorescent light.
There has been a significant increase in many areas from melanoma, something which argues against sunlight being the primary issue as it has not significantly changed in the last few decades. For instance, consider this data from Norway’s cancer registry on malignant melanoma.
Here is the data from that last bullet point, showing that melanoma deaths have been either flat or increasing for decades, depending on age group. Keep in mind that since the mid-1900s people have been spending more time indoors overall, and buying more and more sunscreen products, over this entire interval. The data comes from Norway, where it is known there has been no increase in UV radiation in this timespan.
I can’t find sunscreen data going back to the mid-20th century, but sales patterns since the 2000s, industry projections, and common sense all point to the same basic observation: commercial sunscreen use has increased ever since they were invented, and the trend is likely to continue.
Perhaps we’ve forgotten that humans, like other creatures, have had to manage their sunlight exposure for a hell of a lot longer than commercial sunscreens have been available for purchase. How do animals, including humans, regulate sun exposure under natural conditions? Well… they use their senses, including the largest sensory organ of the body: skin. In fact, our species evolved to be largely hairless, which has probably given us especially sensitive skin-based sensory abilities.
Modern humans have scrambled our ability to use this exquisite sensory system by covering it up with chemicals. Should you look to the future, and keep relying on the newer, “better” product innovations? Or should you look to the past, and come back to your senses?
Under pre-industrial conditions, humans in traditional societies had a couple of basic, common sense options for dealing with intense daytime sun:
Make intermittent use of shade when your senses tell you to (skin gets hot, starts to turn red).
Cover yourself in mud or something, or wear breathable sun-blocking garments.
Today, the “official” strategic advice from medical institutions is that the best approach to human-sunlight interaction is the minimize direct contact of your bodily surfaces with sunlight, at all times and at all costs. Alternative viewpoints are expressed in the two podcasts below. For our purposes in this article, just keep in mind that commercial sunscreens do not block all wavelengths—even with sunscreen, you will still be experiencing some level of oxidative stress in the skin.
To learn more human photobiology and the effects of sunlight:
M&M 221: Regenerative Energy & the Light Inside You | Jack Kruse
M&M 146: Photobiology, Sunlight, Firelight, Incandescent Bulbs vs. LEDs, Mitochondria, Melatonin, Sunscreen & the Optics of the Body | Scott Zimmerman
If your use of sunscreen causes you to stay in intense, direct sunlight for far longer stretches of time than you would otherwise, then you may very well be taking on more overall oxidative stress within your tissues over time, compared to if you used natural sensory feedback and intermittent sun-shade-seeking, rather than multi-hour stint of direct sun exposure while wearing. sunscreen.
So, applying certain molecules to the body’s outer surface can modulate photosensitivity and “sun tolerance,” as does changing the concentration of light-absorbing molecules like melanin within the skin.
What about molecules you absorb into your body? As it turns out, certain medications and food components can affect skin photosensitivity.
Prescription Drugs, Food Components & Skin Photosensitivity
Many different medications can increase dermal photosensitivity, leading to phototoxic or photoallergic reactions when the skin is exposed to ultraviolet (UV) or visible light. Various drugs, ranging from antibiotics to NSAIDs, antihistamines, and cardiovascular medications can have this type of effect.
I discussed this with physician Dr. Brian Kerely on M&M 233. He brought up the example of the antibiotic doxycycline:
Foods can also change dermal photosensitivity. This effect is linked to psoralen and other photosensitizing compounds found in some plants. For example, citrus fruits like lemons, limes, oranges, and grapefruits contain furocoumarins, a family of organic compounds that includes psoralen, which can have this type of effect.
Limes are apparently the most notorious. "Margarita burn" is where lime juice on the skin, combined with sun exposure, causes burns or hyperpigmentation. Other vegetables that contain psoralens include celery, carrots, figs, and more. Phytophotodermatitis causes redness, burns, blisters, or hyperpigmentation, often in irregular patterns (e.g., drips from lime juice). These effects can happen with ingestion of furocoumarins, not just skin application (the reaction is usually milder with ingestion than direct skin contact).
Grapefruit juice may also enhance photosensitivity, especially when combined with certain medications. (Grapefruit is well-known to strongly modulate liver enzymes, which can affect drug metabolism. More detail in this article).
The question we’re ultimately interested in here is whether certain dietary fats—the omega-6 (n-6) PUFAs found in seed oils, like linoleic acid—can make you more sensitive to sunburn. We anticipated the answer above, by reviewing how PUFAs relate to the oxidative stress potential of cells. We’ve also seen that both natural and artificial sunscreens can modulate sunburn sensitivity when present on or within skin, and that certain drugs and food components can, when ingested, enhance dermal photosensitivity.
Let’s look at one more way that dermal photosensitivity can be modulated before turning to dietary PUFAs directly: topical application of cholesterol and fatty acids, including linoleic acid.
Learn more about skin biology and light:
M&M 104: Benefits & Risks of UV Radiation & Sunlight, Skin Health, Vitamin D, Nitric Oxide, Evolution of Skin Color | Richard Weller
Cholesterol, Linoleic Acid & Skin Photosensitivity
I’m no expert on cosmetics and moisturizers, but there is a massive literature on how different substances, natural and synthetic, affect human skin. The cosmetics industry provides a lot of the funding, as you can imagine. Since we are interested in whether ingesting dietary fatty acids might influence skin photosensitivity, I wanted to first see if PUFAs like linoleic acid can influence UV skin responses when applied topically. I quickly found out that they do, as this study shows.
Before looking at the specific results, recall these key facts:
Endogenously produced ROS or external stimuli like UV light can induce oxidative stress. With enough oxidative stress, ROS start damaging macromolecules our cells need to function—DNA, proteins, and lipids—which can lead to cellular dysfunction.
Severely damaged or dead cells stimulate an inflammation, in which mechanisms are initiated to repair damage, clear out debris, and rejuvenate tissue. Incomplete rejuvenation can result in cosmetic issues like scarring, functional issues like metabolic disruption, or even cell death.
Chronic inflammation underlies many disease states and is a major energy sink—the more energy that’s funneled into metabolically expensive inflammatory processes that aren’t strictly needed, the less energy left over to power other aspects of life, including those we associate with vitality. An increasing number of researchers view cancer itself as a form of chronic metabolic disruption, stimulated in some cases by lipid-based inflammation.
Linoleic acid is the most abundant n-6 PUFA found in seed oils. It is a precursor to inflammatory lipids like prostaglandins. NSAIDs like aspirin inhibit the enzyme COX-2, which prevents the formation of these PUFA-derived lipids (coincidentally, aspirin also has anti-cancer effects).
If linoleic acid is not used by cells to make inflammatory lipids or other effector molecules, it can be incorporated into membranes where it may be subject to lipid peroxidation. When linoleic acid is oxidized in this manner, it produces toxic chemicals like MDA and 4-HNE, which can damage or kill cells, resulting in inflammation by other means.
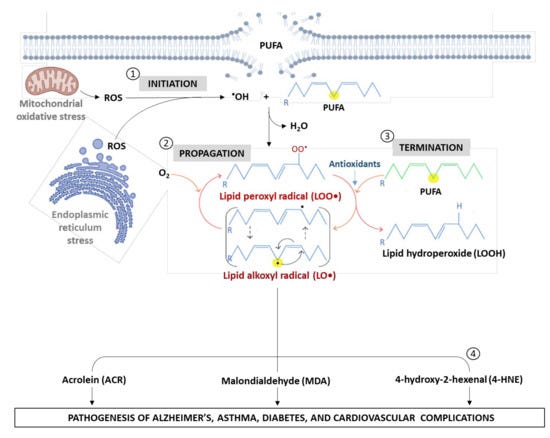
So, there are two separate routes by which a dietary n-6 PUFA like linoleic acid can lead to oxidative stress and inflammation:
A cell can biochemically transform linoleic acid into prostaglandins when needed, using enzymes like COX-2 to do this “on purpose,” when there’s a need to stimulate inflammation (e.g. injury);
Linoleic acid or other PUFAs present in cell membranes can be non-enzymatically oxidized by ROS “on accident,” producing toxins that cause cell damage.
Recall also that compounds with conjugated ring structures, like melanin and synthetic sunscreen molecules, are good at absorbing light. With that in mind, compare the structure of cholesterol vs. linoleic acid:
Without knowing anything else, and knowing very little about physical chemistry, it should be intuitive that each molecule will be impacted by light differently. Cholesterol is composed of conjugated rings; linoleic acid is “slender,” with multiple C-C double bonds at its “elbow.” What does your gut instinct tell you about the general effect each molecule might have on light-exposed skin?
Let’s check our instincts against what’s been observed experimentally…
Topical Cholesterol vs. Linoleic Acid: Basic Results
This study tested the effects of three major lipid ingredients in moisturizers—cholesterol, linoleic acid, and a synthetic ceramide—on human skin responses to UV. The work was done by academic dermatology researchers in Korea, without apparent industry funding.
Some basic facts about lipids and skin penetration, from the paper:
Percutaneous penetration of a topically applied material is mainly determined by its molecular size and lipid solubility. The chemicals with a molecular weight <500 and with adequate lipid solubility are known to penetrate the intact human skin. In the barrier-disrupted skin, even larger chemicals and increased amounts can penetrate. Many of the lipid ingredients in moisturizers have molecular weights <500, as follows: cholesterol, 387; linoleic acid, 280; stearic acid, 284; and palmitic acid, 256. Synthetic ceramides used in moisturizers have various molecular weight around 500–900. Therefore, it is probable that these lipids can penetrate the human skin and reach the inner viable layers, especially under barrier-disrupted conditions. It has been demonstrated that mixtures of cholesterol, linoleic acid, palmitic acid, and ceramides penetrate into the inner nucleated layers in hairless mice skin.
Basic set-up: moisturizers containing one of these three lipid compounds was applied to the skin of healthy human volunteers (11-12 per group). After two days of application, skin was UV irradiated, and samples taken 24-hours later for analysis. They documented physical changes in cells using microscopy and measured expression level changes in markers of skin metabolism and inflammation.
The basic findings for cholesterol vs. linoleic acid included:
Cholesterol significantly decreased the degree of dermal inflammatory infiltrates, compared to controls (linoleic acid did not).
Cholesterol significantly suppressed UV-induced expression of genes associated with inflammation. Linoleic acid tended to have the opposite effect.
The basic conclusion was that, “Topical cholesterol can protect the barrier-disrupted skin against UV-induced damage, while linoleic acid alone has the potential to aggravate the damage.”
Here’s what some of the data looked like, showing distinct patterns of change for skin treated with topical cholesterol vs. linoleic acid:
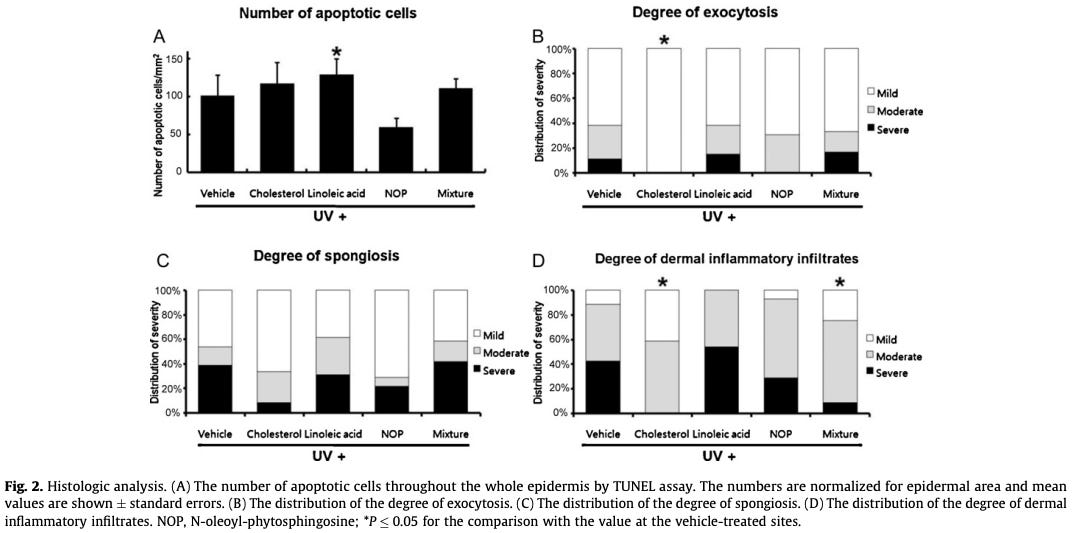
Bottom line: applying cholesterol, linoleic acid, or other lipids to the skin can modulate how UV light exposure affects skin tissue. Linoleic acid appears to promote UV sensitivity and inflammation.
Since fatty compounds like linoleic acid can modulate dermal photosensitivity when applied topically to skin, can the PUFA content of your diet actually play a role in your propensity for sunburn? We know that the PUFA density of cell membranes clearly influences the total potential level of oxidative stress that can ensue if ROS or other noxious stimuli trigger lipid peroxidation.
The next question is: how much of an effect does dietary fat intake have on the fatty acid composition of our lipids?
Does dietary fat composition significantly influence the fatty acid profile of our lipids? Or does the body maintain relatively stable fatty acid profiles, despite changes in fat intake patterns?
Effects of Diet on Cell Membrane & Adipose Tissue Composition
Let’s jump to the punchline:
Dietary fatty acid composition, especially the balance and amount of PUFAs, directly shapes the PUFA density in cell membranes and fat tissue. The effect is strongest for n-3 and n-6 PUFAs, with cell membranes and adipose tissue reflecting dietary intake, particularly when n-3 PUFA intake is low.
If you’re familiar with my writing and podcast, you may already know that humans cannot synthesize PUFAs de novo. Our biology is set up to function best with a balanced intake ratio of n-6 and n-3 PUFAs, as they compete for access to the same enzymes and often subserve opposing functions, such as inflammation initiation (n-6) vs. resolution (n-3).
In contrast, the human body does synthesize saturated and monounsaturated fatty acids. This is why the lipid composition of your cell membranes is particularly sensitive to PUFA intake compared to saturated and monounsaturated fat intake. The specific fatty acid profile of membranes differs across tissues and cell types, but is influenced by dietary fat composition to some extent.
Here’s a snapshot of what is known about the fatty acid content of cell membranes and adipose tissue:
Effects on Cell Membrane Fatty Acid Composition
PUFA Sensitivity: Cell membrane composition is most responsive to dietary n-3 and n-6 PUFAs, especially the n-3/n-6 ratio. Membranes maintain relatively stable levels of saturated and monounsaturated fats despite dietary changes, but PUFA content can shift significantly with dietary intake.
Threshold Effect: When dietary n-3 PUFAs are <10% of total PUFA intake, membrane PUFA composition closely mirrors the diet. Above that threshold, further increases in dietary n-3 have a diminished effect on membrane composition.
Functional Impact: Changes in membrane PUFA content can alter membrane fluidity and the function of membrane proteins, affecting cellular processes and potentially influencing disease risk.
Effects on Fat Tissue (Adipose) Fatty Acid Composition
Dietary Reflection: The fatty acid profile of adipose tissue, especially for essential fatty acids, strongly reflects dietary intake. This includes both the types and proportions of PUFAs consumed.
Adipose Tissue as a Biomarker: Adipose tissue fatty acid profiles can serve as biomarkers for dietary fat intake, particularly for PUFAs.
(For more detail on those points, try this, this, or this).
Seed Oils & Sunburn: Can cutting back really help?
To summarize important points so far:
Sunburn arises from tissue inflammation triggered by high levels of oxidative stress in the skin.
Our skin is naturally plastic and can adapt itself to external light conditions by modulating melanin levels, which changes how much sunlight can be tolerated before sunburn is generated.
Ingesting certain medications and foods can alter dermal photosensitivity.
Topically applying fatty compounds to the skin can alter UV sensitivity and skin inflammation. This includes linoleic acid, the most abundant PUFA in seed oils.
Lipid peroxidation of membrane PUFAs produces cellular toxins which can drive oxidative stress and cell death.
The PUFA density of cell membranes and adipose tissue is sensitive to dietary fat intake patterns.
Given those things, why wouldn’t the n-6 PUFA content of skin cells play some role in modulating sunburn sensitivity?
As far as I’m aware, there have been no large, randomized controlled trials (RCTs) that carefully measure tissue PUFA load, control dietary fat intake, and assess sunburn propensity and tissue oxidative stress levels in a controlled manner. It’s very unlikely that such a study will be done. Are large RCTs always a strict requirement for us to believe something, or make lifestyle decisions regarding our health?
Consider this: there were never any large RCTs that directly proved smoking cigarettes causes cancer in humans. Instead, there was was an accumulation of observational results in the human literature, together with mechanistic studies in animals, plus some common sense, including direct observations by physicians interacting with patients. Smoke contains toxic chemicals. Toxic chemicals break stuff in your cells, eventually leading to dysfunction or cell death. If something contains toxins and you’re exposed to those toxins over a long period of time, something’s going to break at some point.
(Side note: the toxins in cigarette smoke—chemicals like acrolein, 4-HNE, and MDA—are all also produced from lipid peroxidation of PUFAs. If someone tries to argue that lipid peroxidation of membrane PUFAs doesn’t pose any health risk, ask them to explain this brute chemical fact.)
In my conversation with Dr. Brian Kerely on M&M 233, he explained his mindset this way:
Dr. Brian Kerely: I think that mechanism plus result is a real understanding of something, right? People joke about that. Do we need RCTs for parachutes? No, because you can understand the mechanism and see the result, right?
[At a different time, commenting on why there aren’t more RCTs or other studies on seed oils (a claim people commonly make)]
BK: There are more studies out there about oxidative stress and lipid peroxidation than you could ever read—existing right now, links on PubMed. We should all be skeptical, but there's a certain point where you just have to make a decision for your life. So I'm a skeptical on the degree [of certain things related to seed oils], but I've made the decision for myself [to cut out seed oils.] I would recommend for somebody to be a low omega-6 diet. And I'm not waiting to have the perfect RCT on it.
Part of what he was getting at here is that there is a voluminous scientific literature about how lipid peroxidation of PUFAs produces toxins that drive all kinds of metabolic dysregulation and disease states. Many of those those studies do not mention seed oils or diet at all. This is why on social media, Dr. Kerely often shares diagrams like this, from scientific papers:
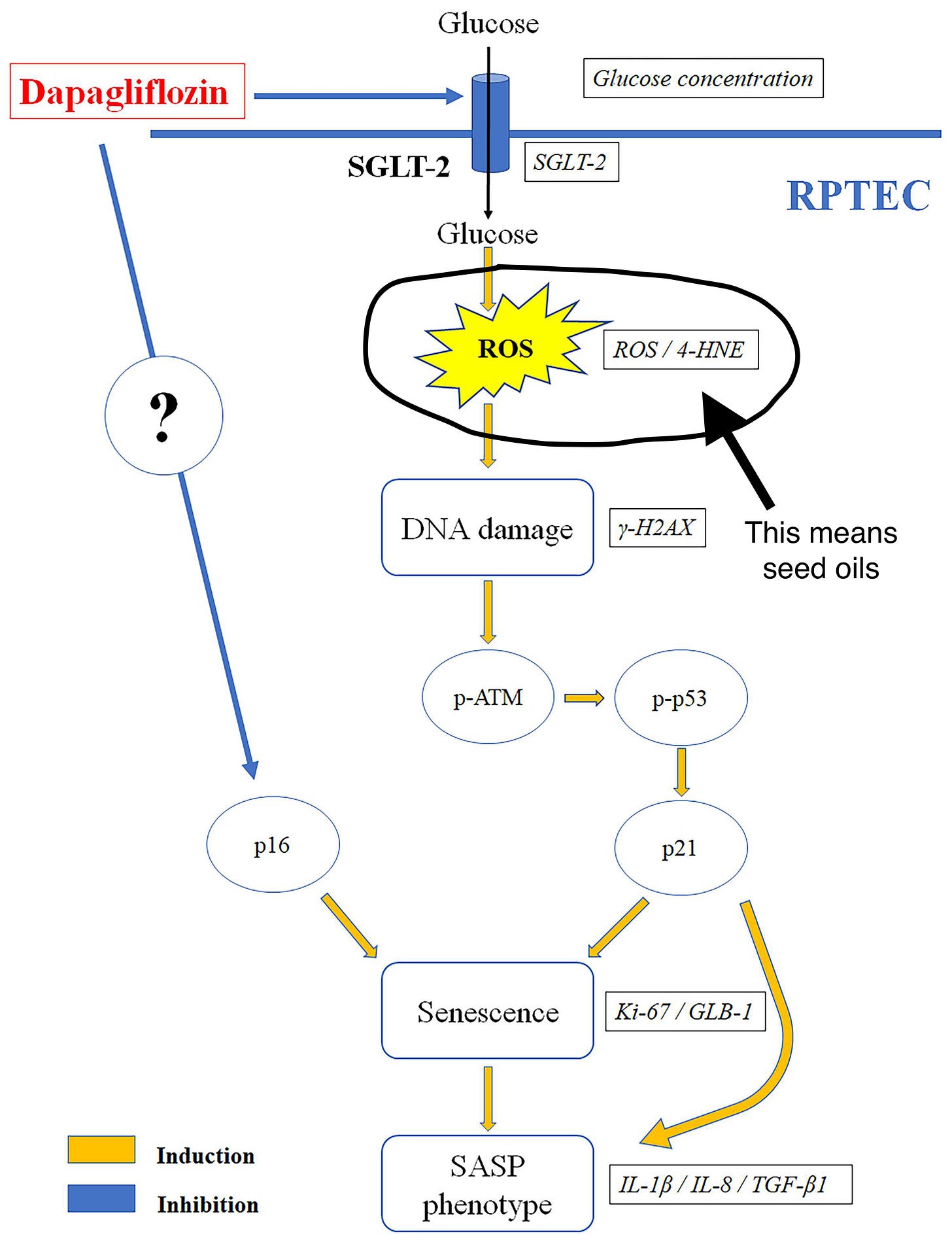
This paper is about a drug called “dapagliflozin,” which can apparently prevent glucose-induced cellular senescence in kidney cells. Terms like “seed oils,” “PUFAs,” and “fat” do not appear anywhere in the paper, which has nothing directly to do with diet. However, in the diagram above, the pathway they depict—the very pathway leading to cell senescence, which this drug prevents—depends on a step labelled, “ROS / 4-HNE.” As we already reviewed, 4-HNE is a toxic chemical generated from non-enzymatic oxidation of membrane PUFAs. There are thousands of papers like this, where no explicit connection is made to PUFAs of any kind. You have to have the relevant background knowledge to realize that the connection is there.
None of us can actually take a “wait and see” approach to our diet. You can certainly update your behavior if and when compelling clinical evidence comes out, clearly suggesting you should start of stop consuming something. But what are you going to eat… today? Why or why not?
A couple DIY ways to investigate this question yourself. The first is quick-and-dirty, and the second requires dietary change, probably for months (perhaps several or more).
Quick-and-dirty: Take some oils, rub them on different patches of skin, and see what happens. Maybe you let some soybean oil, high in linoleic acid, soak into a small patch of skin on your arm. Rub some other oil, low in PUFAs, on the other arm or an adjacent patch of skin. Does one patch start to turn red, feel hot, and eventually sunburn before the other? If you’re sufficiently curious and impatient, try something like this and let us know what happens. Or…
Extended Dietary & sunlight exposure change: This is what I’ve done. If you’ve been fairly diligent on cutting back on dietary n-6 PUFAs, which requires limiting seed oil intake, then your PUFA load should be lower, especially if you used to eat lots of this stuff. Now, try sun exposure. Use common sense. Again: no one is saying that cutting seed oils leads to sun immunity, which is silly. Do you notice a difference in how much sun you can soak before burning, or your tan?
As we reviewed above, changing your dietary PUFA intake will result in changes to the PUFA content of your lipids. However, this is not going to happen overnight. In animals, clear changes in the PUFA content of membranes and adipose tissue are typically measured after several weeks of dietary shift. In humans, this would be many months or even years of time. As described on M&M 200 by lipidomics researchers, many aspects of lipid biology happen slowly, in biological terms:
Some of these trials I've read, [they say:] “we did 10 weeks of an obesity diet for a patient, and 10 weeks of a normal diet, then we looked at plasma levels of omega-6 and omega-3. And, okay—is 10 weeks a lifetime? No. I mean, this is glacial stuff. It doesn't happen overnight. It takes decades. So decades of exposure may cause these risks. So I think some of these RCTs, you just can't do it long enough to see the effects.
—Dr. Tim Yeatman, M&M 200
If you eat anything like the standard American diet, or are simply not too concerned with what’s in what you eat, then you’re eating lots of omega-6 PUFAs from seed oils and processed foods. Prehistoric humans are estimated to have gotten roughly a couple percent of their calories from these fats. Today, people are routinely getting 10%, 20%, or even more of their calories from linoleic acid alone.
So, let’s say you’re such a person. If you started diligently avoiding seed oils and omega-6 PUFAs from any source today, your cell membranes and adipose tissue will not be much different tomorrow. It’s going to take time for lipid profiles to shift. Skin cells divide and get replaced more quickly than many other cells, so it’s presumably not going to take years for those membrane lipid profiles to change if your PUFA intake drastically decreases.
What have I done?
Roughly speaking, I’ve been diligent about minimizing seed oil consumption for a couple of years now. My diet wasn’t trash before that, but it’s really been the last couple of years that I’ve consistently avoided omega-6 PUFAs. I’m fair-skinned and have always burned easily—not a red-head who bursts into flames with any strong sun exposure, but someone who will burn under high UV with more than, say, 30 minutes of continuous exposure.
Once upon a time, like most people, I followed standard dermatological dogma, and almost always applied SPF to my skin, at least when I knew I’d be outside for extended periods. Some years ago, I became biased towards mineral and away from chemical sunscreens. By last summer I was less keen on using SPF at all and recognized the benefits of getting common sense levels of direct and indirect sun. I mostly reserved mineral sunscreens for days at the beach, on a boat, etc. I was also avoiding seed oils, but not quite as diligently as I do now.
This year, and in part because of my conversation with and subsequent reading inspired by Jack Kruse, things have changed even more. I’ve been consuming a low omega-6 PUFA diet for at least a couple years now, and sun exposures patterns have changed much more. In short, I am not using SPF of any kind, getting midday sun exposure for at least 1-2 stints per day (usually 30+ minutes, with intermittent shade). If I can’t get morning/evening sun, using an indoor red/NIR light instead.
The sample size is n=1, but I’m happy to report my results: no sunburns, with more melanin than I’ve ever seen before.
Have I held every other variable constant? Is this a randomized controlled trial? Is it rigorous science? No. But it is my life. Decisions need to be made.
Learn more about seed oils, sunlight, and oxidative stress:












Brilliant. Thank you. I am grateful to be a beneficiary of your hard work. 🙏