Imaging Study Compares Effects of Psilocybin vs. SSRI on Brains of Depressed Patients
Breakdown of a new brain imaging study comparing the effects of psilocybin and an SSRI in depressed patients
A recent study performed brain imaging scans on patients with treatment-resistant depression, after treatment protocols combining psychotherapy with either psilocybin (found in magic mushrooms) or the conventional antidepressant escitalopram (an SSRI). Researchers looked at depression severity at multiple timepoints following each treatment, as well as measures of functional connectivity between different brain networks.
What did they find and what does it tell us?
The Problem
Depression is a highly prevalent, debilitating, and costly mental health condition which has become worse during the COVID-19 pandemic. Despite the heavy burden this places on society, the best antidepressant drugs we have, such as Selective Serotonin Reuptake Inhibitors (SSRIs), show “modest efficacy, non-negligible side effects, discontinuation problems and high relapse rates.”
Many patients prescribed conventional antidepressants also end up taking additional psychoactive prescription drugs, like antipsychotics. It is becoming more and more common to continue taking these drugs for years at a time. I’ve previously written about this trend:

Over the past 15 years, several clinical trials have independently shown that psilocybin therapy can lead to significant improvements in depressive symptoms, often enduring for many months. Despite the flurry of medical research on psilocybin and other psychedelics in recent years, we still know very little about what these drugs are doing to patients’ brains, or how this compares to conventional SSRI treatments.
The Study: Effect of Psilocybin on Depression Scores
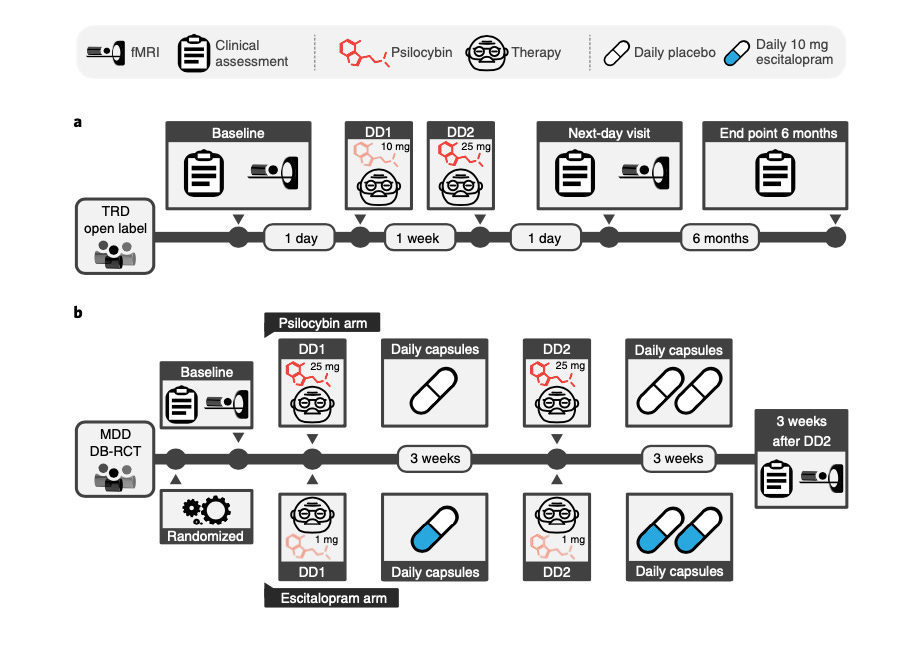
The new study analyzed brain imaging data collected from two previous clinical trials using psilocybin therapy in depressed patients. The first of those was an open-label trial, where patients were not blind to the treatment condition—they knew they were getting psilocybin. In this trial, patients received two successive psilocybin doses: a low-medium dose (10 mg) followed by a high dose (25 mg), separated by one week. Each dose was accompanied by a therapy session, with brain scans (fMRI) conducted before the first dose and after the second dose.
25 mg will induce a full-blown psychedelic experience in most people. 10 mg is a lower but would still produce distinct psychoactive effects. These are not microdoses.
All patients in this trial had treatment-resistant depression (TRD), meaning they had tried conventional treatments with minimal success. To measure depression, researchers used the “Beck Depression Inventory” (BDI) score. High scores (e.g. >30) indicate strong depression. Low scores indicate lesser symptom severity. This was measured before the first psilocybin dose, as well as 1 week, 3 months, and 6 months after the second dose.
Here are the basic results for how two successive doses of psilocybin (with therapy) impacts depression scores in patients with treatment-resistant depression:
Panel A shows depression scores before and after psilocybin treatment. Lower numbers are good here, i.e. milder depression. Notice two things:
Depression scores after psilocybin treatment are all lower, on average, compared to the baseline period.
The variance in depression scores is wider for the three post-psilocybin time points.
So psilocybin therapy had an antidepressant effect, on average, at each time point, but did not work equally well for all patients. Some patients saw significant improvements starting within a week and lasting for six months. A few showed mild reductions in depression severity at one week, which faded by three months. Several others displayed a range of different symptom improvement patterns.
The other clinical data they analyzed came from a double-blind, randomized control trial comparing psilocybin to escitalopram, an SSRI antidepressant. In this trial, patients were randomly assigned to two groups, one getting two separate 25 mg doses of psilocybin and the other getting daily doses of escitalopram. No patients knew for sure whether they were getting psilocybin.
While this trial was similar to the first in many ways to—both involved two doses with therapy, plus brain imaging—the timing and dosages used were somewhat different. Patients had been diagnosed with Major Depressive Disorder (MDD), but not Treat-Resistant Depression (TRD) as in the first trial.
Here’s how the two study designs compare:
Using the same measure of depression severity as the open-label study, here’s what they observed for the effects of psilocybin and the SSRI escitalopram on depression severity:
Notice some basic patterns:
Both psilocybin and escitalopram were associated with lower depression scores at two, four, and six weeks after treatment, but the psilocybin group showed significantly lower scores (milder depression) than the SSRI at each time point.
Baseline depression was somewhat lower in this group compared to the open-label trial, as were median depression scores after treatment. This may be because the first trial looked at patients with treatment-resistant depression.
The antidepressant effect is variable for both psilocybin and escitalopram—not all patients respond in the same way. The overall anti-depression effect is larger for psilocybin than escitalopram in this trial, and a bit stronger than in the open-label trial.
So far, so good. But they also conducted fMRI brain scans of all these patients, which allowed researchers to understand how functional measures of brain physiology compared across these conditions. This is the component of the paper that’s new and nudges forward our understanding of how psilocybin elicits antidepressant effects in the brain.
The Study: Background Neuroscience
Before going over the results, let’s go over some concepts needed to understand what the data is showing.
First, they used functional magnetic resonance imaging (fMRI). This imaging technique looks at functional, not anatomical, features of the brain. It does this by measuring an aspect of brain metabolism. Like muscles, neurons are metabolically expensive cells. When neurons become more active they need to consume more fuel (glucose). Blood flow dynamically changes within the brain to deliver this as needed, which changes blood oxygenation levels. fMRI measures this. It’s not a direct measure of the electrochemical activity of individual neurons, but an indirect measure of metabolic changes associated with the activity of large groups of neurons.
Using fMRI, neuroscientists can map out different networks in the brain based on the patterns of metabolic change they observe. Brain networks are composed of multiple brain regions that tend to “work together.” A given network might be comprised brain regions showing systematically correlated changes in metabolic activity when a person performs a certain type of cognitive task, or goes into specific emotional states.
This study primarily looked at three brain networks, each implicated in depression:
Default mode network (DMN): Associated with introspection and self-referential thinking, this network tends to be hyperactive in depressed patients and may be involved in negative rumination about one’s self. Both meditation and psychedelics have been associated with decreases in DMN activity (learn more here).
Executive Network (EN): Associated with various aspects of “cognitive control.” This includes switching the focus of attention, e.g. from internal thoughts or emotions to external events in the world. Attentional switching is often impaired in depression.
Salience Network (SN): Similar to the EN, this network is also tied to cognitive control. SN is specifically associated with shifting attention to salient aspects of our experience—the parts that are especially important and “pop-out” from the rest, such as a sudden loud noise or Substack article you’re reading with excitement.
Why these three brain networks?
In addition to being implicated in depression, these networks have some of the highest serotonin 2A receptor (5-HT2A) densities in the brain. This is the “psychedelic receptor”—the one required for the major hallucinogenic effects of classic psychedelics like psilocybin, LSD, and DMT.
You can learn more about this receptor from my conversation with Dr. Charles Nichols, in M&M #2:
The last concept to understand is functional connectivity. Again, this is not the same as physical, anatomical connectivity (although these two things are related). Functional connectivity is a measure of how much different brain networks “talk” to one another, or to themselves. When there’s a lot of cross-talk between brain networks, they have higher functional connectivity. When there is not a lot of cross-talk between networks, they have lower functional connectivity.
The level of functional connectivity between brain networks can be measured with a combination of brain imaging and statistical techniques. When there is a high degree of functional connectivity between different networks in the brain, they are said to be more integrated. When there is a low degree of functional connectivity between different brain networks, the brain is more modular—each network is sort of like an island, functionally isolated from other networks.
Here’s what a functional map of the default mode network (DMN), obtained from fMRI, can look like:
And here’s an anatomical map of the fibers connecting the various regions of the DMN, seen from looking down through the top of the brain:
Again, networks like the DMN include multiple regions located in different parts of the brain. Lots of nerve fibers physically connect these region to each other, as well as to other brain networks. These anatomical connections enable functional cross-talk between regions, but the networks themselves are defined in functional terms, based on patterns of brain activity.
The Study: Effects of Psilocybin of Brain Functional Connectivity
Recall: in the first, open-label trial, patients with treatment-resistant depression were given two doses of psilocybin with therapy. After six months, patients showed a wide range of responses to the treatment, with the overall effect being lower depression scores. How did patterns of functional connectivity change before and after treatment?
In the left panel, we see the distribution of a metric (Q) that tells us about brain modularity. Higher Q values indicate a more modular brain—distinct brain networks are behaving more like isolated islands, with low functional connectivity linking them together. When brain networks engage in elevated levels of cross-talk, this modularity metric goes down and the brain is more “integrated.”
After the psilocybin treatment, brain modularity decreased for the majority of patients, indicating more functional connectivity between brain networks. On average, the level of recruitment of the default mode network (DMN) decreased in patients, while it’s level of integration with the executive and salience networks increased (panel D).
Panels B-C are important because they relate these changes in functional brain modularity to the decreases in depression severity observed across patients. This helps start to make sense of why individual patients responded in different ways. They observed a significant correlation between these two variables: patients with the lowest brain modularity scores post-treatment tended to have the lowest depression scores. In other words, the patients with greater levels of improvement in their symptoms tended to have higher levels of integration between the DMN, executive, and salience networks.
What about the imaging results for the patients in the double-blind, randomized trial comparing psilocybin to an SSRI?
The overall result is similar for the psilocybin treatment group: a decrease in brain modularity that correlates with the level of symptom improvement. What’s most interesting here is the comparison to the SSRI group. As a reminder, both the psilocybin and SSRI treatment groups saw an overall improvement in depression scores, with the psilocybin group showing somewhat greater improvement.
In terms of the functional brain imaging results, however, these two groups were distinct. The group that received the SSRI (escitalopram) did not show a decrease in brain modularity, and there was no significant correlation between changes in depression severity and functional measures of global brain integration.
One important caveat here is to notice that the psilocybin and SSRI treatment groups differed in their brain modularity scores before treatment, at baseline (panels A vs. D). The escitalopram (SSRI) group had more patients with low brain modularity scores at baseline. If that group had looked more like the psilocybin group, the post-treatment changes in brain modularity may not have been as distinct between treatment groups. Independent replication of this result is needed.
The study has not been without criticism, including from other researchers in psychedelic science. I recently talked to Dr. Manoj Doss about it in M&M #74 (towards the end).
What Do These Results Mean?
This study combined clinical measures of depression in response to drug-therapy interventions with functional measures of brain activity. This allowed researchers to correlate changes in functional connectivity to clinical outcomes. Do these functional changes cause the improvements in depression that were observed? We can’t be sure (“correlation does not prove causation”). But now we have a basic foothold into some of the functional changes within the brain associated with psilocybin’s robust antidepressant effects.
The brain imaging results also make intuitive sense based on what we know about what these brain networks are doing in both normal and depressed people. In addition, they make sense in terms of how these networks behave in other mental disorders where people often get “stuck” maladaptive patterns of thought and behavior.
The Executive Network and Salience Network have been associated with tasks requiring cognitive flexibility such as learning and task switching; impaired functioning of these networks have been reported in depression and other disorders exhibiting cognitive inflexibility such as autism spectrum disorder and obsessive–compulsive disorder.
One striking result was the difference in functional brain changes observed for psilocybin compared to escitalopram. Both drugs were associated with an improvement in depression but only psilocybin was associated with changes in functional brain network connectivity. This shows that the same overall clinical outcome (lower depression scores) from two treatments can involve distinct physiological changes within the brain. Just because two treatments are associated with similar clinical outcomes does not mean they are interchangeable. I suspect that the functional brain changes observed for psilocybin may relate the strength of the antidepressant effect, or how long it endures, compared to SSRIs.
The psilocybin-associated changes in functional in the default mode and other networks are also fairly straightforward to interpret in light of the way patients describe the subjective effects:
Psilocybin seems to increase the brain’s ability to visit a broader state space, both acutely and after psilocybin therapy in patients who are depressed, as shown here. Moreover, this ‘liberating’ action of psilocybin is paralleled by subjective reports of ‘emotional release’ as well as subacute increases in behavioral optimism, cognitive flexibility and psychological flexibility after taking a psychedelic drug.
It’s also telling that these functional changes were observed in brain networks with the high densities of the “psychedelic receptor” 5-HT2A, using high doses of psilocybin. This may hint at the role that the psychedelic experience plays in the strength of clinical outcomes.
It’s still an open question whether or not the hallucinogenic effects of psychedelics are strictly required for their therapeutic effects. Researchers are divided on the potential for developing non-hallucinogenic drug variants of classic psychedelics, as I recently discussed with Dr. Gul Dolen in M&M #62.
There is still a lot to learn about how, exactly, psychedelics are exerting their profound effects on consciousness and mental health. There’s much more that we don’t know compared to what we do know.
To learn more about the topics covered in this essay, try these episodes of the Mind & Matter podcast:
Chris Timmermann: DMT (Dimethyltryptamine), Brain Waves & the Psychedelic Experience | #25
Hamilton Morris: Psychoactive Drugs, Psychedelics, Society & Hamilton's Pharmacopeia | #6
David Olson: Psychedelics, Psychoplastogens, Microdosing, Mental Health, Brain Chemistry, Creating Novel Drugs | #46
Manoj Doss: Psychoactive Drugs & Memory (Sedatives, Stimulants, Cannabinoids, Psychedelics, MDMA) | #74